Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies
- PMID: 23383169
- PMCID: PMC3561263
- DOI: 10.1371/journal.pone.0055375
Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies
Abstract
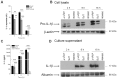
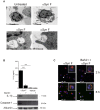
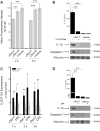
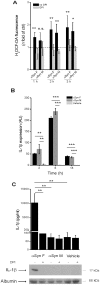
Parkinson's disease (PD) is one of the most common neurodegenerative diseases. It is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta of the brain. Another feature is represented by the formation in these cells of inclusions called Lewy bodies (LB), principally constituted by fibrillar α-synuclein (αSyn). This protein is considered a key element in the aetiology of a group of neurodegenerative disorders termed synucleinopathies, which include PD, but the cellular and molecular mechanisms involved are not completely clear. It is established that the inflammatory process plays a crucial role in the pathogenesis and/or progression of PD; moreover, it is known that aggregated αSyn, released by neurons, activates microglia cells to produce pro-inflammatory mediators, such as IL-1β. IL-1β is one of the strongest pro-inflammatory cytokines; it is produced as an inactive mediator, and its maturation and activation requires inflammasome activation. In particular, the NLRP3 inflammasome is activated by a wide variety of stimuli, among which are crystallized and particulate material. In this work, we investigated the possibility that IL-1β production, induced by fibrillar αSyn, is involved the inflammasome activation. We demonstrated the competence of monomeric and fibrillar αSyn to induce synthesis of IL-1β, through TLR2 interaction; we found that the secretion of the mature cytokine was a peculiarity of the fibrillated protein. Moreover, we observed that the secretion of IL-1β involves NLRP3 inflammasome activation. The latter relies on the phagocytosis of fibrillar αSyn, followed by increased ROS production and cathepsin B release into the cytosol. Taken together, our data support the notion that fibrillar αSyn, likely released by neuronal degeneration, acts as an endogenous trigger inducing a strong inflammatory response in PD.
Conflict of interest statement
Figures

References
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, et al. (2000) Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54: S21–23. - PubMed
-
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211. - PubMed
-
- Gasser T (2005) Genetics of Parkinson's disease. Curr Opin Neurol 18: 363–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

