Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death
- PMID: 23383204
- PMCID: PMC3561218
- DOI: 10.1371/journal.pone.0055497
Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death
Abstract
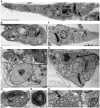
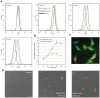
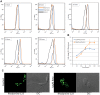
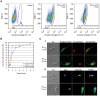
The protozoan parasite Trypanosoma cruzi displays similarities to fungi in terms of its sterol lipid biosynthesis, as ergosterol and other 24-alkylated sterols are its principal endogenous sterols. The sterol pathway is thus a potential drug target for the treatment of Chagas disease. We describe here a comparative study of the growth inhibition, ultrastructural and physiological changes leading to the death of T. cruzi cells following treatment with the sterol biosynthesis inhibitors (SBIs) ketoconazole and lovastatin. We first calculated the drug concentration inhibiting epimastigote growth by 50% (EC(50)/72 h) or killing all cells within 24 hours (EC(100)/24 h). Incubation with inhibitors at the EC(50)/72 h resulted in interesting morphological changes: intense proliferation of the inner mitochondrial membrane, which was corroborated by flow cytometry and confocal microscopy of the parasites stained with rhodamine 123, and strong swelling of the reservosomes, which was confirmed by acridine orange staining. These changes to the mitochondria and reservosomes may reflect the involvement of these organelles in ergosterol biosynthesis or the progressive autophagic process culminating in cell lysis after 6 to 7 days of treatment with SBIs at the EC(50)/72 h. By contrast, treatment with SBIs at the EC(100)/24 h resulted in rapid cell death with a necrotic phenotype: time-dependent cytosolic calcium overload, mitochondrial depolarization and reservosome membrane permeabilization (RMP), culminating in cell lysis after a few hours of drug exposure. We provide the first demonstration that RMP constitutes the "point of no return" in the cell death cascade, and propose a model for the necrotic cell death of T. cruzi. Thus, SBIs trigger cell death by different mechanisms, depending on the dose used, in T. cruzi. These findings shed new light on ergosterol biosynthesis and the mechanisms of programmed cell death in this ancient protozoan parasite.
Conflict of interest statement
Figures


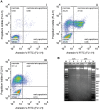
References
-
- Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. - PubMed
-
- Coura JR, Dias JC (2009) Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz 104 Suppl 1: 31–40. - PubMed
-
- Wilkinson SR, Kelly JM (2009) Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med 11: e31. - PubMed
-
- Camargo EP (2009) Perspectives of vaccination in Chagas disease revisited. Mem Inst Oswaldo Cruz 104 Suppl 1: 275–280. - PubMed
-
- Dumonteil E (2009) Vaccine development against Trypanosoma cruzi and Leishmania species in the post-genomic era. Infect Genet Evol 9: 1075–1082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

