Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD
- PMID: 23384071
- PMCID: PMC3570361
- DOI: 10.1186/1465-9921-14-15
Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD
Abstract
Background: Rhinovirus (RV) is a major cause of chronic obstructive pulmonary disease (COPD) exacerbations, and primarily infects bronchial epithelial cells. Immune responses from BECs to RV infection are critical in limiting viral replication, and remain unclear in COPD. The objective of this study is to investigate innate immune responses to RV infection in COPD primary BECs (pBECs) in comparison to healthy controls.
Methods: Primary bronchial epithelial cells (pBECs) from subjects with COPD and healthy controls were infected with RV-1B. Cells and cell supernatant were collected and analysed using gene expression microarray, qPCR, ELISA, flow cytometry and titration assay for viral replication.
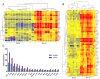
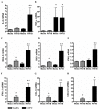
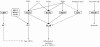
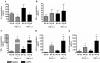
Results: COPD pBECs responded to RV-1B infection with an increased expression of antiviral and pro-inflammatory genes compared to healthy pBECs, including cytokines, chemokines, RNA helicases, and interferons (IFNs). Similar levels of viral replication were observed in both disease groups; however COPD pBECs were highly susceptible to apoptosis. COPD pBECs differed at baseline in the expression of 9 genes, including calgranulins S100A8/A9, and 22 genes after RV-1B infection including the signalling proteins pellino-1 and interleukin-1 receptor associated kinase 2. In COPD, IFN-β/λ1 pre-treatment did not change MDA-5/RIG-I and IFN-β expression, but resulted in higher levels IFN-λ1, CXCL-10 and CCL-5. This led to reduced viral replication, but did not increase pro-inflammatory cytokines.
Conclusions: COPD pBECs elicit an exaggerated pro-inflammatory and antiviral response to RV-1B infection, without changing viral replication. IFN pre-treatment reduced viral replication. This study identified novel genes and pathways involved in potentiating the inflammatory response to RV in COPD.
Figures
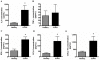

References
-
- Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, Busse WW. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–627. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

