Monogenic hyperinsulinemic hypoglycemia: current insights into the pathogenesis and management
- PMID: 23384201
- PMCID: PMC3573904
- DOI: 10.1186/1687-9856-2013-3
Monogenic hyperinsulinemic hypoglycemia: current insights into the pathogenesis and management
Abstract
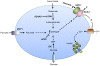
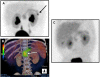
Hyperinsulinism (HI) is the leading cause of persistent hypoglycemia in children, which if unrecognized may lead to development delays and permanent neurologic damage. Prompt recognition and appropriate treatment of HI are essential to avoid these sequelae. Major advances have been made over the past two decades in understanding the molecular basis of hyperinsulinism and mutations in nine genes are currently known to cause HI. Inactivating KATP channel mutations cause the most common and severe type of HI, which occurs in both a focal and a diffuse form. Activating mutations of glutamate dehydrogenase (GDH) lead to hyperinsulinism/hyperammonemia syndrome, while activating mutations of glucokinase (GK), the "glucose sensor" of the beta cell, causes hyperinsulinism with a variable clinical phenotype. More recently identified genetic causes include mutations in the genes encoding short-chain 3-hydroxyacyl-CoA (SCHAD), uncoupling protein 2 (UCP2), hepatocyte nuclear factor 4-alpha (HNF-4α), hepatocyte nuclear factor 1-alpha (HNF-1α), and monocarboyxlate transporter 1 (MCT-1), which results in a very rare form of HI triggered by exercise. For a timely diagnosis, a critical sample and a glucagon stimulation test should be done when plasma glucose is < 50 mg/dL. A failure to respond to a trial of diazoxide, a KATP channel agonist, suggests a KATP defect, which frequently requires pancreatectomy. Surgery is palliative for children with diffuse KATPHI, but children with focal KATPHI are cured with a limited pancreatectomy. Therefore, distinguishing between diffuse and focal disease and localizing the focal lesion in the pancreas are crucial aspects of HI management. Since 2003, 18 F-DOPA PET scans have been used to differentiate diffuse and focal disease and localize focal lesions with higher sensitivity and specificity than more invasive interventional radiology techniques. Hyperinsulinism remains a challenging disorder, but recent advances in the understanding of its genetic basis and breakthroughs in management should lead to improved outcomes for these children.
Figures

References
-
- Stanley CA, De Leon DD. Monogenic Hyperinsulinemic Hypoglycemia Disorders. 1. Basel: Karger; 2012.
-
- Monogenic Disorders of Insulin Secretion. Congenital Hyperinsulinism and Neonatal Diabetes March 15–16, 2012 Faculty Synopses. Pediatr Diabetes. 2012;13(4):344–368.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

