Red blood cell polymorphism and susceptibility to Plasmodium vivax
- PMID: 23384621
- PMCID: PMC3728992
- DOI: 10.1016/B978-0-12-407826-0.00002-3
Red blood cell polymorphism and susceptibility to Plasmodium vivax
Abstract
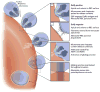
Resistance to Plasmodium vivax blood-stage infection has been widely recognised to result from absence of the Duffy (Fy) blood group from the surface of red blood cells (RBCs) in individuals of African descent. Interestingly, recent studies from different malaria-endemic regions have begun to reveal new perspectives on the association between Duffy gene polymorphism and P. vivax malaria. In Papua New Guinea and the Americas, heterozygous carriers of a Duffy-negative allele are less susceptible to P. vivax infection than Duffy-positive homozygotes. In Brazil, studies show that the Fy(a) antigen, compared to Fy(b), is associated with lower binding to the P. vivax Duffy-binding protein and reduced susceptibility to vivax malaria. Additionally, it is interesting that numerous studies have now shown that P. vivax can infect RBCs and cause clinical disease in Duffy-negative people. This suggests that the relationship between P. vivax and the Duffy antigen is more complex than customarily described. Evidence of P. vivax Duffy-independent red cell invasion indicates that the parasite must be evolving alternative red cell invasion pathways. In this chapter, we review the evidence for P. vivax Duffy-dependent and Duffy-independent red cell invasion. We also consider the influence of further host gene polymorphism associated with malaria endemicity on susceptibility to vivax malaria. The interaction between the parasite and the RBC has significant potential to influence the effectiveness of P. vivax-specific vaccines and drug treatments. Ultimately, the relationships between red cell polymorphisms and P. vivax blood-stage infection will influence our estimates on the population at risk and efforts to eliminate vivax malaria.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Blood Group Antigen Gene Mutation Database. from http://www.ncbi.nlm.nih.gov/projects/gv/rbc/xslcgi.fcgi?cmd=bgmut/summary. - PubMed
-
- Abdalla S, Weatherall DJ, Wickramasinghe SN, Hughes M. The anaemia of P. falciparum malaria. Br J Haematol. 1980;46:171–183. - PubMed
-
- Adams JH, Hudson DE, Torii M, Ward GE, Wellems TE, Aikawa M, et al. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 085406/WT_/Wellcome Trust/United Kingdom
- AI089686/AI/NIAID NIH HHS/United States
- AI46919/AI/NIAID NIH HHS/United States
- TW007377/TW/FIC NIH HHS/United States
- AI 075416/AI/NIAID NIH HHS/United States
- TW007872/TW/FIC NIH HHS/United States
- R21 AI093922/AI/NIAID NIH HHS/United States
- R01 TW007872/TW/FIC NIH HHS/United States
- D43 TW007377/TW/FIC NIH HHS/United States
- R01 AI046919/AI/NIAID NIH HHS/United States
- R01 AI075416/AI/NIAID NIH HHS/United States
- AI093922/AI/NIAID NIH HHS/United States
- U19 AI089686/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

