Diarrhoea due to small bowel diseases
- PMID: 23384804
- PMCID: PMC3621726
- DOI: 10.1016/j.bpg.2012.11.013
Diarrhoea due to small bowel diseases
Abstract
Small intestinal diseases are a common, though often overlooked cause of diarrhoeal illness. Fully 1% of the Caucasian population are affected by coeliac disease and a substantial portion of children living in poverty in the developing world are affected by environmental enteropathy. These are but two examples of the many diseases that cause mucosal injury to the primary digestive and absorptive organ in our body. While diarrhoea may be a common, though not universally seen symptom of small bowel mucosal disease, the consequent malabsorption can lead to substantial malnutrition and nutrient deficiencies. The small intestine, unlike the colon, has been relatively inaccessible, and systematic evaluation is often necessary to identify and treat small intestinal mucosal diseases that lead to diarrhoea. Immunodeficiency states, including HIV enteropathy, adult autoimmune enteropathy, drug-associated enteropathy, and tropical sprue continue to occur and require specific therapy. All patients with severe diarrhoea or diarrhoea associated with features suggestive of malabsorption may have a disease of the small intestinal mucosa that requires careful evaluation and targeted management.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

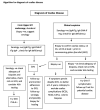
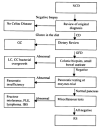







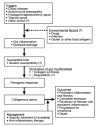

References
-
- Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of coeliac disease in the United States. Am J Gastroenterol Oct. 2012;107(10):1538–44. - PubMed
-
- Green PH, Cellier C. Celiac disease. N Engl J Med. 2007 Oct 25;357(17):1731–43. - PubMed
-
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med. 2006 Apr;119(4):e9–14. (355) - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

