Glutamate and tumor-associated epilepsy: glial cell dysfunction in the peritumoral environment
- PMID: 23385090
- PMCID: PMC3664257
- DOI: 10.1016/j.neuint.2013.01.027
Glutamate and tumor-associated epilepsy: glial cell dysfunction in the peritumoral environment
Abstract
Seizures are a serious and debilitating co-morbidity of primary brain tumors that affect most patients, yet their etiology is poorly understood. In many CNS pathologies, including epilepsy and brain injury, high levels of extracellular glutamate have been implicated in seizure generation. It has been shown that gliomas release neurotoxic levels of glutamate through their high expression of system xc-. More recently it was shown that the surrounding peritumoral cortex is spontaneously hyperexcitable. In this review, we discuss how gliomas induce changes in the surrounding environment that may further contribute to elevated extracellular glutamate and tumor-associated seizures. Peritumoral astrocytes become reactive and lose their ability to remove glutamate, while microglia, in response to signals from glioma cells, may release glutamate. In addition, gliomas increase blood brain barrier permeability, allowing seizure-inducing serum components, including glutamate, into the peritumoral region. These factors, working together or alone, may influence the frequency and severity of tumor-associated epilepsy.
Keywords: Astrocytes; Glioma; Gliosis; Glutamate; Hyperexcitability; Seizures.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
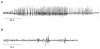
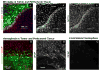
References
-
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. - PubMed
-
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47:11–22. - PubMed
-
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

