Self-assembly, surface activity and structure of n-octyl-β-D-thioglucopyranoside in ethylene glycol-water mixtures
- PMID: 23385232
- PMCID: PMC3588041
- DOI: 10.3390/ijms14023228
Self-assembly, surface activity and structure of n-octyl-β-D-thioglucopyranoside in ethylene glycol-water mixtures
Abstract
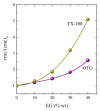
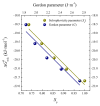
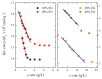
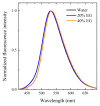
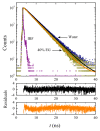
The effect of the addition of ethylene glycol (EG) on the interfacial adsorption and micellar properties of the alkylglucoside surfactant n-octyl-β-D-thioglucopyranoside (OTG) has been investigated. Critical micelle concentrations (cmc) upon EG addition were obtained by both surface tension measurements and the pyrene 1:3 ratio method. A systematic increase in the cmc induced by the presence of the co-solvent was observed. This behavior was attributed to a reduction in the cohesive energy of the mixed solvent with respect to pure water, which favors an increase in the solubility of the surfactant with EG content. Static light scattering measurements revealed a decrease in the mean aggregation number of the OTG micelles with EG addition. Moreover, dynamic light scattering data showed that the effect of the surfactant concentration on micellar size is also controlled by the content of the co-solvent in the system. Finally, the effect of EG addition on the microstructure of OTG micelles was investigated using the hydrophobic probe Coumarin 153 (C153). Time-resolved fluorescence anisotropy decay curves of the probe solubilized in micelles were analyzed using the two-step model. The results indicate a slight reduction of the average reorientation time of the probe molecule with increasing EG in the mixed solvent system, thereby suggesting a lesser compactness induced by the presence of the co-solvent.
Figures




References
-
- Ramadan M.S., Evans D.F., Lumry R. Why micelles form in water and hydrazine—A reexamination of the origins of hydrophobicity. J. Phys. Chem. 1983;87:4538–4543.
-
- Evans D.F., Miller D.D. Organized Solutions and Their Manifestations in Polar Solvents. In: Friberg S.E., Lindman B., editors. Organized Solutions: Surfactants in Science and Technology. Vol. 44. Marcel Dekker Inc; New York, NY, USA: 1992. pp. 33–45.
-
- Sedov I.A., Stolov M.A., Solomonov B.N. Solvophobic effects and relationships between the Gibbs energy and enthalpy for the salvation process. J. Phys. Org. Chem. 2011;24:1088–1094.
-
- Ray A. Solvophobic interactions and micelle formation in structure forming nonaqueous solvents. Nature. 1971;231:313–314. - PubMed
-
- Cantú L., Corti M., Degiorgio V., Hoffmann H., Ulbrichts W. Nonionic micelles in mixed water-glycerol solvent. J. Colloid Interface Sci. 1987;116:384–389.
LinkOut - more resources
Full Text Sources
Other Literature Sources

