Effects of Aging and Hypercholesterolemia on Oxidative Stress and DNA Damage in Bone Marrow Mononuclear Cells in Apolipoprotein E-deficient Mice
- PMID: 23385237
- PMCID: PMC3588046
- DOI: 10.3390/ijms14023325
Effects of Aging and Hypercholesterolemia on Oxidative Stress and DNA Damage in Bone Marrow Mononuclear Cells in Apolipoprotein E-deficient Mice
Abstract
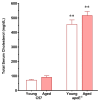
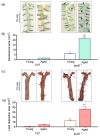
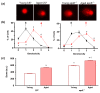
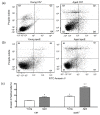
Recent evidence from apolipoprotein E-deficient (apoE-/-) mice shows that aging and atherosclerosis are closely associated with increased oxidative stress and DNA damage in some cells and tissues. However, bone marrow cells, which are physiologically involved in tissue repair have not yet been investigated. In the present study, we evaluated the influence of aging and hypercholesterolemia on oxidative stress, DNA damage and apoptosis in bone marrow cells from young and aged apoE-/- mice compared with age-matched wild-type C57BL/6 (C57) mice, using the comet assay and flow cytometry. The production of both superoxide and hydrogen peroxide in bone marrow cells was higher in young apoE-/- mice than in age-matched C57 mice, and reactive oxygen species were increased in aged C57 and apoE-/- mice. Similar results were observed when we analyzed the DNA damage and apoptosis. Our data showed that both aging and hypercholesterolemia induce the increased production of oxidative stress and consequently DNA damage and apoptosis in bone marrow cells. This study is the first to demonstrate a functionality decrease of the bone marrow, which is a fundamental extra-arterial source of the cells involved in vascular injury repair.
Figures


Similar articles
-
Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis.Biol Res. 2018 Sep 5;51(1):33. doi: 10.1186/s40659-018-0182-7. Biol Res. 2018. PMID: 30185234 Free PMC article.
-
Protective effect of sildenafil on the genotoxicity and cytotoxicity in apolipoprotein E-deficient mice bone marrow cells.Lipids Health Dis. 2016 May 27;15:100. doi: 10.1186/s12944-016-0268-6. Lipids Health Dis. 2016. PMID: 27229150 Free PMC article.
-
Endogenous female sex hormones delay the development of renal dysfunction in apolipoprotein E-deficient mice.Lipids Health Dis. 2014 Nov 25;13:176. doi: 10.1186/1476-511X-13-176. Lipids Health Dis. 2014. PMID: 25422135 Free PMC article.
-
Hypercholesterolemia promotes early renal dysfunction in apolipoprotein E-deficient mice.Lipids Health Dis. 2011 Nov 26;10:220. doi: 10.1186/1476-511X-10-220. Lipids Health Dis. 2011. PMID: 22117541 Free PMC article.
-
Endothelial dysfunction in the apolipoprotein E-deficient mouse: insights into the influence of diet, gender and aging.Lipids Health Dis. 2011 Nov 14;10:211. doi: 10.1186/1476-511X-10-211. Lipids Health Dis. 2011. PMID: 22082357 Free PMC article. Review.
Cited by
-
The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species.Antioxidants (Basel). 2020 Aug 25;9(9):786. doi: 10.3390/antiox9090786. Antioxidants (Basel). 2020. PMID: 32854347 Free PMC article.
-
Dioclea violacea lectin ameliorates oxidative stress and renal dysfunction in an experimental model of acute kidney injury.Am J Transl Res. 2015 Dec 15;7(12):2573-88. eCollection 2015. Am J Transl Res. 2015. PMID: 26885258 Free PMC article.
-
Sildenafil ameliorates oxidative stress and DNA damage in the stenotic kidneys in mice with renovascular hypertension.J Transl Med. 2014 Feb 6;12:35. doi: 10.1186/1479-5876-12-35. J Transl Med. 2014. PMID: 24502628 Free PMC article.
-
Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis.Biol Res. 2018 Sep 5;51(1):33. doi: 10.1186/s40659-018-0182-7. Biol Res. 2018. PMID: 30185234 Free PMC article.
-
Sildenafil ameliorates biomarkers of genotoxicity in an experimental model of spontaneous atherosclerosis.Lipids Health Dis. 2013 Aug 28;12:128. doi: 10.1186/1476-511X-12-128. Lipids Health Dis. 2013. PMID: 23981672 Free PMC article.
References
-
- Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. - PubMed
-
- Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2003;21:361–370. - PubMed
-
- McEwen J.E., Zimniak P., Mehta J.L., Shmookler Reis R.J. Molecular pathology of aging and its implications for senescent coronary atherosclerosis. Curr. Opin. Cardiol. 2005;20:399–406. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

