Diagnosing Mild Cognitive Impairment (MCI) in clinical trials: a systematic review
- PMID: 23386579
- PMCID: PMC3586181
- DOI: 10.1136/bmjopen-2012-001909
Diagnosing Mild Cognitive Impairment (MCI) in clinical trials: a systematic review
Abstract
Objective: To describe how criteria for amnestic Mild Cognitive Impairment (aMCI) have been operationalised in randomised controlled clinical trials (RCTs).
Design: Systematic review.
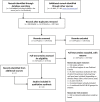
Information sources: EMBASE, PubMed and PSYCHInfo were searched from their inception to February 2012. Electronic clinical trial registries were also searched (February 2012).
Study selection: RCTs were included where participant selection was made using Petersen et al-defined aMCI. There was no restriction on intervention type or the outcome tested.
Data extraction: For each trial, we extracted information on study design, demographics, exclusion criteria and the operationalisation strategy for the five aMCI diagnostic criteria including: (1) memory complaint, (2) normal general cognitive function, (3) memory impairment, (4) no functional impairment and (5) no dementia.
Results: 223 articles and 278 registered trials were reviewed, of which 22 met inclusion criteria. Various methods were applied for operationalising aMCI criteria resulting in variability in participant selection. Memory complaint and assessment of general cognitive function were the most consistently measured criteria. There was large heterogeneity in the neuropsychological methods used to determine memory impairment. It was not possible to assess the impact of these differences on case selection accuracy for dementia prediction. Further limitations include selective and unclear reporting of how each of the criteria was measured.
Conclusions: The results highlight the urgent need for a standardised approach to map aMCI. Lack of uniformity in clinical diagnosis, however, is not exclusively a problem for MCI but also for other clinical states such as dementia including Alzheimer's disease, Lewy Body, frontotemporal or vascular dementia. Defining a uniform approach to MCI classification, or indeed for any classification concept within the field of dementia, should be a priority if further trials are to be undertaken in the older aged population based on these concepts.
Figures
References
-
- Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov 2003;2:646–53 - PubMed
-
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8 - PubMed
-
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous