Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode
- PMID: 23386604
- PMCID: PMC3605637
- DOI: 10.1074/jbc.M112.442053
Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode
Abstract
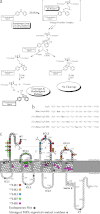
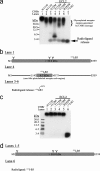
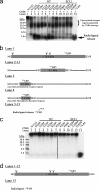
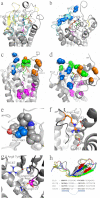
Breakthroughs in G protein-coupled receptor structure determination based on crystallography have been mainly obtained from receptors occupied in their transmembrane domain core by low molecular weight ligands, and we have only recently begun to elucidate how the extracellular surface of G protein-coupled receptors (GPCRs) allows for the binding of larger peptide molecules. In the present study, we used a unique chemoselective photoaffinity labeling strategy, the methionine proximity assay, to directly identify at physiological conditions a total of 38 discrete ligand/receptor contact residues that form the extracellular peptide-binding site of an activated GPCR, the angiotensin II type 1 receptor. This experimental data set was used in homology modeling to guide the positioning of the angiotensin II (AngII) peptide within several GPCR crystal structure templates. We found that the CXC chemokine receptor type 4 accommodated the results better than the other templates evaluated; ligand/receptor contact residues were spatially grouped into defined interaction clusters with AngII. In the resulting receptor structure, a β-hairpin fold in extracellular loop 2 in conjunction with two extracellular disulfide bridges appeared to open and shape the entrance of the ligand-binding site. The bound AngII adopted a somewhat vertical binding mode, allowing concomitant contacts across the extracellular surface and deep within the transmembrane domain core of the receptor. We propose that such a dualistic nature of GPCR interaction could be well suited for diffusible linear peptide ligands and a common feature of other peptidergic class A GPCRs.
Figures


References
-
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

