Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons
- PMID: 23386808
- PMCID: PMC3560102
- DOI: 10.3389/fnins.2013.00003
Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons
Abstract
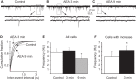
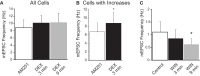
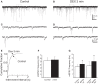
Glucocorticoids rapidly regulate synaptic input to neuroendocrine cells in the hypothalamic paraventricular nucleus (PVN) by inducing the retrograde release of endogenous messengers. Here we investigated the rapid effects of dexamethasone (DEX) on excitatory synaptic input to feeding-related, preautonomic PVN neurons using whole-cell patch-clamp recordings. In ∼50% of identified gastric-related preautonomic PVN neurons, DEX elicited a biphasic synaptic response characterized by an initial rapid and transient increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs), followed by a decrease in mEPSC frequency within 9 min; remaining cells displayed only a decrease in mEPSC frequency. The late-phase decrease in mEPSC frequency was mimicked by the cannabinoid receptor agonists anandamide (AEA) and WIN 55,212-2, and it was blocked by the CB1 receptor antagonist AM251. The biphasic DEX effect was mimicked by AEA. The early increase in mEPSCs was mimicked by activation of transient receptor potential vanilloid type 1 (TRPV1) receptors with capsaicin and by activation of TRPV4 receptors with 4-α-PDD. The increase was reduced, but not blocked, by selective TRPV1 antagonists and in TRPV1 knockout mice; it was blocked completely by the broad-spectrum TRPV antagonist ruthenium red and by combined application of selective TRPV1 and TRPV4 antagonists. The DEX effects were prevented entirely by intracellular infusion of the G-protein inhibitor, GDPβS. Thus, DEX biphasically modulates synaptic glutamate onto a subset of gastric-related PVN neurons, which is likely mediated by induction of a retrograde messenger. The effect includes a TRPV1/4 receptor-mediated transient increase and subsequent CB1 receptor-mediated suppression of glutamate release. Multiphasic modulation of glutamate input to PVN neurons represents a previously unappreciated complexity of control of autonomic output by glucocorticoids and endogenous cannabinoids.
Keywords: cannabinoid; paraventricular nucleus; vanilloid.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

