The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas
- PMID: 23386906
- PMCID: PMC3564248
- DOI: 10.7150/jca.5112
The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas
Abstract
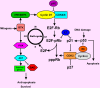
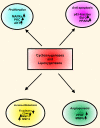
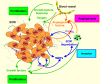
Head and neck squamous cell carcinoma (HNSCC) is a complex tissue that contains tumor cells and the surrounding stroma, which is populated by different types of mesenchymal cells and the extracellular matrix (ECM). Collectively, they are referred to as the tumor microenvironment (TME). Recent studies have shown that TME has a more profound influence on the growth and metastasis of HNSCC than was previously appreciated. Because carcinoma-associated fibroblasts (CAFs) are frequently observed in the stroma of the tumor, this review focuses on the potential role of tumor-CAFs interactions in progression of HNSCC. Tumor-CAFs crosstalk enhances the production of growth factors, cytokines, chemokines, matrix metalloproteinases (MMPs), and inflammatory mediators, which eventually facilitates tumor growth. In fact, factors and cells that do not support tumor growth are usually down regulated or mitigated in TME. Therefore TME may determine the fate of the tumors at the site of invasion and metastasis. For tumor cells that survive at these sites, stromal activation may serve to establish a supportive tumor stroma, fostering the outgrowth of the metastatic cells. The concept of tumor-stromal interactions and microenvironmental niche has profound consequences in tumor growth and metastasis and therefore, it's understanding will open up new strategies for the diagnosis, prognosis and therapy of HNSCC.
Keywords: CCL12.; CXCR4; Cancer associated fibroblasts (CAFs); Cycloxygenase-2 (COX-2); Head and neck cancer; Matrix metalloproteinases (MMPs).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Duvvuri U, Myers JN. Cancer of the head and neck is the sixth most common cancer worldwide. Curr Probl Surg. 2009;46:114–117. - PubMed
-
- Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86:104–114. - PubMed
-
- Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37:1–10. - PubMed
-
- van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; present concepts of management. Oral Oncol. 2010;46:423–425. - PubMed
-
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

