A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis
- PMID: 23387374
- PMCID: PMC3761190
- DOI: 10.1111/bjd.12266
A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis
Abstract
Background: Tofacitinib (CP-690,550) is a novel Janus kinase inhibitor in development as an oral formulation for the treatment of several inflammatory diseases including psoriasis.
Objectives: This phase 2a study aimed to assess the efficacy, systemic safety, local tolerability and systemic pharmacokinetics of topical tofacitinib in mild-to-moderate plaque psoriasis.
Methods: Two tofacitinib ointment formulations were evaluated in this multicentre, double-blind, vehicle-controlled trial (NCT01246583). Seventy-one patients were randomized 2 : 1 : 2 : 1 to 2% tofacitinib ointment 1, vehicle 1, 2% tofacitinib ointment 2 and vehicle 2, each administered twice daily for 4 weeks to a single fixed 300 cm(2) treatment area containing a target plaque with or without one or more nontarget plaques and normal skin.
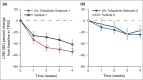
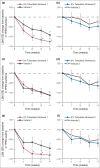
Results: The primary endpoint of percentage change from baseline in the Target Plaque Severity Score at week 4 demonstrated statistically significant improvement for ointment 1 [least squares mean (LSM) -54.4%] vs. vehicle 1 (LSM -41.5%), but not ointment 2 (LSM -24.2%) vs. vehicle 2 (LSM -17.2%). Secondary endpoints (target plaque area and Itch Severity Item) improved similarly for tofacitinib ointment vs. corresponding vehicle. Adverse event (AE) occurrence was similar across treatment groups. All AEs were mild or moderate and none were serious or led to subject discontinuation. One application-site AE (erythema) was reported. Tofacitinib mean systemic exposure was minimal and was greater for ointment 1 than for ointment 2.
Conclusions: Tofacitinib ointment 1 was well tolerated and efficacious compared with vehicle for the treatment of plaque psoriasis. Further study of topical tofacitinib for psoriasis treatment is warranted.
© The Authors © 2013 Pfizer Inc BJD © 2013 British Association of Dermatologists.
Figures


Comment in
-
Systemics to topicals in psoriasis: the unfilled need.Br J Dermatol. 2013 Jul;169(1):2-3. doi: 10.1111/bjd.12434. Br J Dermatol. 2013. PMID: 23834112 No abstract available.
References
-
- Gudjonsson JE, Elder JT. Psoriasis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller A, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th edn. New York: McGraw-Hill Medical; 2012. pp. 197–232.
-
- Papp K, Gulliver W, Lynde C, et al. Canadian guidelines for the management of plaque psoriasis: overview. J Cutan Med Surg. 2011;15:210–19. - PubMed
-
- Williams SC. New biologic drugs get under the skin of psoriasis. Nat Med. 2012;18:638. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

