An automated high-throughput counting method for screening circulating tumor cells in peripheral blood
- PMID: 23387387
- PMCID: PMC3586433
- DOI: 10.1021/ac400193b
An automated high-throughput counting method for screening circulating tumor cells in peripheral blood
Abstract
Enumeration of circulating tumor cells (CTCs) has proved valuable for early detection and prognosis in cancer treatment. This paper describes an automated high-throughput counting method for CTCs based on microfluidics and line-confocal microscopy. Peripheral blood was directly labeled with multiple antibodies, each conjugated with a different fluorophore, pneumatically pumped through a microfluidic channel, and interrogated by a line-confocal microscope. On the basis of the fluorescence signals and labeling schemes, the count of CTCs was automatically reported. Due to the high flow rate, 1 mL of whole blood can be analyzed in less than 30 min. We applied this method in analyzing CTCs from 90 stage IV breast cancer patient samples and performed a side-by-side comparison with the results of the CellSearch assay, which is the only method approved by the U.S. Food and Drug Administration at present for enumeration of CTCs. This method has a recovery rate for cultured breast cancer cells of 94% (n = 9), with an average of 1.2 counts/mL of background level of detected CTCs from healthy donors. It detected CTCs from breast cancer patients ranging from 15 to 3375 counts/7.5 mL. Using this method, we also demonstrate the ability to enumerate CTCs from breast cancer patients that were positive for Her2 or CD44(+)/CD24(-), which is a putative cancer stem cell marker. This automated method can enumerate CTCs from peripheral blood with high throughput and sensitivity. It could potentially benefit the clinical diagnosis and prognosis of cancer.
Figures

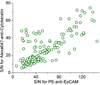
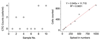
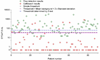
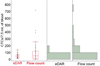
References
-
- Steeg PS. Nat. Med. 2006;12:895–904. - PubMed
-
- Chaffer CL, Weinberg RA. Science. 2011;331:1559–1564. - PubMed
-
- Fidler I. Nat. Rev. Cancer. 2003;3:453–458. - PubMed
-
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, RiethmUller G, Klein CA. Cancer Cell. 2008;13:58–68. - PubMed
-
- Ashworth TR. Aust. Med. J. 1869;14:2.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

