Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with Ph-negative ALL: results of a prospective study (ALL MRD2002 Study)
- PMID: 23388549
- PMCID: PMC3574830
- DOI: 10.1186/1756-8722-6-14
Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with Ph-negative ALL: results of a prospective study (ALL MRD2002 Study)
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) for patients with Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) in first complete remission (CR1) is much more intensive than multi-agent combined chemotherapy, although allogeneic HSCT is associated with increased morbidity and mortality when compared with such chemotherapy. Minimal residual disease (MRD) status has been proven to be a strong prognostic factor for adult patients with Ph-negative ALL.
Methods: We investigated whether MRD status in adult patients with ALL is useful to decide clinical indications for allogeneic HSCT. We prospectively monitored MRD after induction and consolidation therapy in adult patients with Ph-negative ALL.
Results: Of 110 adult ALL patients enrolled between July 2002 and August 2008, 101 were eligible, including 59 Ph-negative patients. MRD status was assessed in 43 patients by the detection of major rearrangements in TCR and Ig and the presence of chimeric mRNA. Thirty-nine patients achieved CR1, and their probabilities of 3-year overall survival and disease-free survival (DFS) were 74% and 56%, respectively. Patients who were MRD-negative after induction therapy (n = 26) had a significantly better 3-year DFS compared with those who were MRD-positive (n = 13; 69% vs. 31%, p = 0.004). All of 3 patients who were MRD-positive following consolidation chemotherapy and did not undergo allogeneic HSCT, relapsed and died within 3 years after CR.
Conclusions: These results indicate that MRD monitoring is useful for determining the clinical indications for allogeneic HSCT in the treatment of ALL in CR1.
Figures
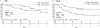

References
-
- Hoelzer D, Thiel E, Loffler H, Buchner T, Ganser A, Heil G, Koch P, Freund M, Diedrich H, Ruhl H. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–131. - PubMed
-
- Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, Bueso-Ramos CE, Pierce S, Shan J, Koller C. et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. - DOI - PubMed
-
- Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N. et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. - DOI - PubMed
-
- Lazarus HM, Richards SM, Chopra R, Litzow MR, Burnett AK, Wiernik PH, Franklin IM, Tallman MS, Cook L, Buck G. et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006;108:465–472. doi: 10.1182/blood-2005-11-4666. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

