Endoplasmic reticulum stress sensing in the unfolded protein response
- PMID: 23388626
- PMCID: PMC3578356
- DOI: 10.1101/cshperspect.a013169
Endoplasmic reticulum stress sensing in the unfolded protein response
Abstract
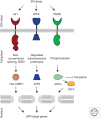
Secretory and transmembrane proteins enter the endoplasmic reticulum (ER) as unfolded proteins and exit as either folded proteins in transit to their target organelles or as misfolded proteins targeted for degradation. The unfolded protein response (UPR) maintains the protein-folding homeostasis within the ER, ensuring that the protein-folding capacity of the ER meets the load of client proteins. Activation of the UPR depends on three ER stress sensor proteins, Ire1, PERK, and ATF6. Although the consequences of activation are well understood, how these sensors detect ER stress remains unclear. Recent evidence suggests that yeast Ire1 directly binds to unfolded proteins, which induces its oligomerization and activation. BiP dissociation from Ire1 regulates this oligomeric equilibrium, ultimately modulating Ire1's sensitivity and duration of activation. The mechanistic principles of ER stress sensing are the focus of this review.
Figures


Comment in
-
Endoplasmic reticulum stress and fungal pathogenesis converge.Virulence. 2014 Feb 15;5(2):331-3. doi: 10.4161/viru.28051. Epub 2014 Feb 6. Virulence. 2014. PMID: 24504109 Free PMC article. No abstract available.
References
-
- Abravaya K, Myers MP, Murphy SP, Morimoto RI 1992. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6: 1153–1164 - PubMed
-
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K 2008. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct 33: 75–89 - PubMed
-
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL 2004. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and insigs. J Biol Chem 279: 52772–52780 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
