Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes
- PMID: 23388639
- PMCID: PMC3581956
- DOI: 10.1073/pnas.1222861110
Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes
Abstract
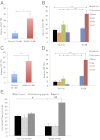
Myelodysplastic syndromes (MDS) are a group of disorders characterized by variable cytopenias and ineffective hematopoiesis. Hematopoietic stem cells (HSCs) and myeloid progenitors in MDS have not been extensively characterized. We transplanted purified human HSCs from MDS samples into immunodeficient mice and show that HSCs are the disease-initiating cells in MDS. We identify a recurrent loss of granulocyte-macrophage progenitors (GMPs) in the bone marrow of low risk MDS patients that can distinguish low risk MDS from clinical mimics, thus providing a simple diagnostic tool. The loss of GMPs is likely due to increased apoptosis and increased phagocytosis, the latter due to the up-regulation of cell surface calreticulin, a prophagocytic marker. Blocking calreticulin on low risk MDS myeloid progenitors rescues them from phagocytosis in vitro. However, in the high-risk refractory anemia with excess blasts (RAEB) stages of MDS, the GMP population is increased in frequency compared with normal, and myeloid progenitors evade phagocytosis due to up-regulation of CD47, an antiphagocytic marker. Blocking CD47 leads to the selective phagocytosis of this population. We propose that MDS HSCs compete with normal HSCs in the patients by increasing their frequency at the expense of normal hematopoiesis, that the loss of MDS myeloid progenitors by programmed cell death and programmed cell removal are, in part, responsible for the cytopenias, and that up-regulation of the "don't eat me" signal CD47 on MDS myeloid progenitors is an important transition step leading from low risk MDS to high risk MDS and, possibly, to acute myeloid leukemia.
Conflict of interest statement
Conflict of interest statement: W.W.P., C.Y.P., and I.L.W. filed US Patent Application Serial No. 13/508,319 entitled “Cell Surface Marker Expression in Hematopoietic Stem Cells and Progenitors for the Diagnosis, Prognosis, and Treatment of Myelodysplastic Syndromes;” I.L.W. filed US Patent Application Serial No. 12/321,215 entitled “Methods for Manipulating Phagocytosis Mediated by CD47;” and I.L.W. filed a patent application regarding therapeutic and diagnostic methods for manipulating phagocytosis through calreticulin and low-density lipoprotein-related receptors.
Figures
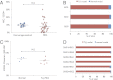
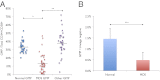

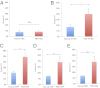
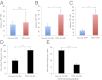
References
-
- Brunning R, et al. Myelodysplastic syndromes/neoplasms. In: Swerdlow S, et al., editors. WHO Classification of Tumours and Haematopoietic and Lymphoid Tissues. Vol 2. Lyon, France: Intl Agency Res Cancer; 2008.
-
- Hofmann WK, Koeffler HP. Myelodysplastic syndrome. Annu Rev Med. 2005;56:1–16. - PubMed
-
- Greenberg P, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

