Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines
- PMID: 23388721
- PMCID: PMC3624395
- DOI: 10.1128/JVI.02628-12
Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines
Abstract
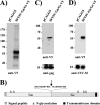
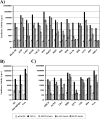
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel bunyavirus that recently emerged in China. Infection with SFTSV is associated with case-fatality rates of up to 30%, and neither antivirals nor vaccines are available at present. Development of antiviral strategies requires the elucidation of virus-host cell interactions. Here, we analyzed host cell entry of SFTSV. Employing lentiviral and rhabdoviral vectors, we found that the Gn/Gc glycoproteins (Gn/Gc) of SFTSV mediate entry into a broad range of human and animal cell lines, as well as human macrophages and dendritic cells. The Gn/Gc proteins of La Crosse virus (LACV) and Rift Valley Fever Virus (RVFV), other members of the bunyavirus family, facilitated entry into an overlapping but not identical range of cell lines, suggesting that SFTSV, LACV, and RVFV might differ in their receptor requirements. Entry driven by SFTSV Gn/Gc was dependent on low pH but did not require the activity of the pH-dependent endosomal/lysosomal cysteine proteases cathepsins B and L. Instead, the activity of a cellular serine protease was required for infection driven by SFTSV and LACV Gn/Gc. Sera from convalescent SFTS patients inhibited SFTSV Gn/Gc-driven host cell entry in a dose-dependent fashion, demonstrating that the vector system employed is suitable to detect neutralizing antibodies. Finally, the C-type lectin DC-SIGN was found to serve as a receptor for SFTSV Gn/Gc-driven entry into cell lines and dendritic cells. Our results provide initial insights into cell tropism, receptor usage, and proteolytic activation of SFTSV and will aid in the understanding of viral spread and pathogenesis.
Figures


References
-
- Walter CT, Barr JN. 2011. Recent advances in the molecular and cellular biology of bunyaviruses. J. Gen. Virol. 92:2467–2484 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

