Vac14 protein multimerization is a prerequisite step for Fab1 protein complex assembly and function
- PMID: 23389034
- PMCID: PMC3611006
- DOI: 10.1074/jbc.M113.453712
Vac14 protein multimerization is a prerequisite step for Fab1 protein complex assembly and function
Abstract
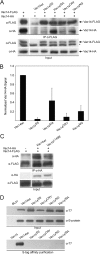
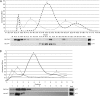
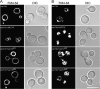
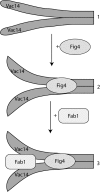
Phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) helps control various endolysosome functions including organelle morphology, membrane recycling, and ion transport. Further highlighting its importance, PtdIns(3,5)P2 misregulation leads to the development of neurodegenerative diseases like Charcot-Marie-Tooth disease. The Fab1/PIKfyve lipid kinase phosphorylates PtdIns(3)P into PtdIns(3,5)P2 whereas the Fig4/Sac3 lipid phosphatase antagonizes this reaction. Interestingly, Fab1 and Fig4 form a common protein complex that coordinates synthesis and degradation of PtdIns(3,5)P2 by a poorly understood process. Assembly of the Fab1 complex requires Vac14/ArPIKfyve, a multimeric scaffolding adaptor protein that coordinates synthesis and turnover of PtdIns(3,5)P2. However, the properties and function of Vac14 multimerization remain mostly uncharacterized. Here we identify several conserved C-terminal motifs on Vac14 required for self-interaction and provide evidence that Vac14 likely forms a dimer. We also show that monomeric Vac14 mutants do not support interaction with Fab1 or Fig4, suggesting that Vac14 multimerization is likely the first molecular event in the assembly of the Fab1 complex. Finally, we show that cells expressing monomeric Vac14 mutants have enlarged vacuoles that do not fragment after hyperosmotic shock, which indicates that PtdIns(3,5)P2 levels are greatly abated. Therefore, our observations support an essential role for the Vac14 homocomplex in controlling PtdIns(3,5)P2 levels.
Figures



References
-
- Ho C. Y., Alghamdi T. A., Botelho R. J. (2012) Phosphatidylinositol 3,5-bisphosphate: no longer the poor PIP2. Traffic 13, 1–8 - PubMed
-
- Ikonomov O. C., Sbrissa D., Mlak K., Shisheva A. (2002) Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 143, 4742–4754 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

