X-ray crystal structure of Escherichia coli RNA polymerase σ70 holoenzyme
- PMID: 23389035
- PMCID: PMC3610985
- DOI: 10.1074/jbc.M112.430900
X-ray crystal structure of Escherichia coli RNA polymerase σ70 holoenzyme
Abstract
Escherichia coli RNA polymerase (RNAP) is the most studied bacterial RNAP and has been used as the model RNAP for screening and evaluating potential RNAP-targeting antibiotics. However, the x-ray crystal structure of E. coli RNAP has been limited to individual domains. Here, I report the x-ray structure of the E. coli RNAP σ(70) holoenzyme, which shows σ region 1.1 (σ1.1) and the α subunit C-terminal domain for the first time in the context of an intact RNAP. σ1.1 is positioned at the RNAP DNA-binding channel and completely blocks DNA entry to the RNAP active site. The structure reveals that σ1.1 contains a basic patch on its surface, which may play an important role in DNA interaction to facilitate open promoter complex formation. The α subunit C-terminal domain is positioned next to σ domain 4 with a fully stretched linker between the N- and C-terminal domains. E. coli RNAP crystals can be prepared from a convenient overexpression system, allowing further structural studies of bacterial RNAP mutants, including functionally deficient and antibiotic-resistant RNAPs.
Figures


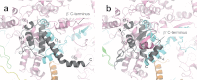


References
-
- Werner F., Grohmann D. (2011) Evolution of multisubunit RNA polymerases in the three domains of life. Nat. Rev. Microbiol. 9, 85–98 - PubMed
-
- Gruber T. M., Gross C. A. (2003) Multiple σ subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57, 441–466 - PubMed
-
- Murakami K. S., Darst S. A. (2003) Bacterial RNA polymerases: the wholo story. Curr. Opin. Struct. Biol. 13, 31–39 - PubMed
-
- Murakami K. S., Masuda S., Darst S. A. (2002) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296, 1280–1284 - PubMed
-
- Vassylyev D. G., Sekine S., Laptenko O., Lee J., Vassylyeva M. N., Borukhov S., Yokoyama S. (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417, 712–719 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

