Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications
- PMID: 23389111
- PMCID: PMC4422342
- DOI: 10.1152/ajpregu.00355.2012
Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications
Abstract
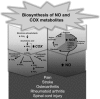
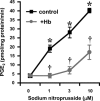
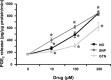
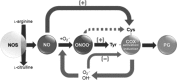
The nitric oxide (NO) and cyclooxygenase (COX) pathways share a number of similarities. Nitric oxide is the mediator generated from the NO synthase (NOS) pathway, and COX converts arachidonic acid to prostaglandins, prostacyclin, and thromboxane A(2). Two major forms of NOS and COX have been identified to date. The constitutive isoforms critically regulate several physiological states. The inducible isoforms are overexpressed during inflammation in a variety of cells, producing large amounts of NO and prostaglandins, which may underlie pathological processes. The cross-talk between the COX and NOS pathways was initially reported by Salvemini and colleagues in 1993, when they demonstrated in a series of in vitro and in vivo studies that NO activates the COX enzymes to produce increased amounts of prostaglandins. Those studies led to the concept that COX enzymes represent important endogenous "receptor" targets for amplifying or modulating the multifaceted roles of NO in physiology and pathology. Since then, numerous studies have furthered our mechanistic understanding of these interactions in pathophysiological settings and delineated potential clinical outcomes. In addition, emerging evidence suggests that the canonical nitroxidative species (NO, superoxide, and/or peroxynitrite) modulate biosynthesis of prostaglandins through non-COX-related pathways. This article provides a comprehensive state-of-the art overview in this area.
Figures


References
-
- Ahlner J, Andersson RG, Torfgard K, Axelsson KL. Organic nitrate esters: clinical use and mechanisms of actions. Pharmacol Rev 43: 351–423, 1991. - PubMed
-
- Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem 274: 842–848, 1999. - PubMed
-
- Bailey JM, Muza B, Hla T, Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res 26: 54–61, 1985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

