Cellular and molecular regulation of vascular permeability
- PMID: 23389236
- PMCID: PMC3786592
- DOI: 10.1160/TH12-09-0678
Cellular and molecular regulation of vascular permeability
Abstract
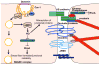
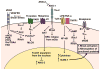
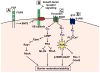
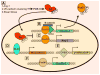
Vascular permeability is a highly coordinated process that integrates vesicular trafficking, complex junctional rearrangements, and refined cytoskeletal dynamics. In response to the extracellular environment, these three cellular activities have been previously assumed to work in parallel to regulate the passage of solutes between the blood and tissues. New developments in the area of vascular permeability, however have highlighted the interdependence between trans- and para-cellular pathways, the cross-communication between adherens and tight junctions, and the instructional role of pericytes on endothelial expression of barrier-related genes. Additionally, significant effort has been placed in understanding the molecular underpinings that contribute to barrier restoration following acute permeability events and in clarifying the importance of context-dependent signaling initiated by permeability mediators. Finally, recent findings have uncovered an unpredicted role for transcription factors in the coordination of vascular permeability and clarified how junctional complexes can transmit signals to the nucleus to control barrier function. The goal of this review is to provide a concise and updated view of vascular permeability, discuss the most recent advances in molecular and cellular regulation, and introduce integrated information on the central mechanisms involved in trans-endothelial transport.
Conflict of interest statement
None declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

