NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche
- PMID: 23389444
- PMCID: PMC3635831
- DOI: 10.1038/nature11847
NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche
Abstract
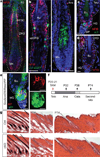
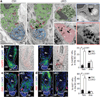
Adult stem cells reside in specialized niches where they receive environmental cues to maintain tissue homeostasis. In mammals, the stem cell niche within hair follicles is home to epithelial hair follicle stem cells and melanocyte stem cells, which sustain cyclical bouts of hair regeneration and pigmentation. To generate pigmented hairs, synchrony is achieved such that upon initiation of a new hair cycle, stem cells of each type activate lineage commitment. Dissecting the inter-stem-cell crosstalk governing this intricate coordination has been difficult, because mutations affecting one lineage often affect the other. Here we identify transcription factor NFIB as an unanticipated coordinator of stem cell behaviour. Hair follicle stem-cell-specific conditional targeting of Nfib in mice uncouples stem cell synchrony. Remarkably, this happens not by perturbing hair cycle and follicle architecture, but rather by promoting melanocyte stem cell proliferation and differentiation. The early production of melanin is restricted to melanocyte stem cells at the niche base. Melanocyte stem cells more distant from the dermal papilla are unscathed, thereby preventing hair greying typical of melanocyte stem cell differentiation mutants. Furthermore, we pinpoint KIT-ligand as a dermal papilla signal promoting melanocyte stem cell differentiation. Additionally, through chromatin-immunoprecipitation with high-throughput-sequencing and transcriptional profiling, we identify endothelin 2 (Edn2) as an NFIB target aberrantly activated in NFIB-deficient hair follicle stem cells. Ectopically induced Edn2 recapitulates NFIB-deficient phenotypes in wild-type mice. Conversely, endothelin receptor antagonists and/or KIT blocking antibodies prevent precocious melanocyte stem cell differentiation in the NFIB-deficient niche. Our findings reveal how melanocyte and hair follicle stem cell behaviours maintain reliance upon cooperative factors within the niche, and how this can be uncoupled in injury, stress and disease states.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

