Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury
- PMID: 23389456
- PMCID: PMC3625838
- DOI: 10.1152/ajprenal.00543.2012
Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury
Abstract
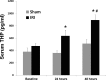
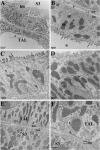
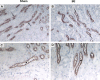
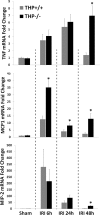
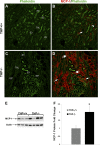
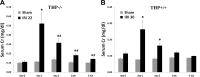
Tamm-Horsfall protein (THP) is a glycoprotein normally targeted to the apical membrane domain of the kidney's thick ascending limbs (TAL). We previously showed that THP of TAL confers protection to proximal tubules against acute kidney injury (AKI) via a possible cross talk between the two functionally distinct tubular segments. However, the extent, timing, specificity, and functional effects of basolateral translocation of THP during AKI remain unclear. Using an ischemia-reperfusion (IRI) model of murine AKI, we show here that, while THP expression in TAL is downregulated at the peak of injury, it is significantly upregulated 48 h after IRI. Confocal immunofluorescence and immunoelectron microscopy reveal a major redirection of THP during recovery from the apical membrane domain of TAL towards the basolateral domain, interstitium, and basal compartment of S3 segments. This corresponds with increased THP in the serum but not in the urine. The overall epithelial polarity of TAL cells does not change, as evidenced by correct apical targeting of Na(+)-K(+)-2Cl cotransporter (NKCC2) and basolateral targeting of Na(+)-K(+)-ATPase. Compared with the wild-type, THP(-/-) mice show a significantly delayed renal recovery after IRI, due possibly to reduced suppression by THP of proinflammatory cytokines and chemokines such as monocyte chemoattractant protein-1 during recovery. Taken together, our data suggest that THP redistribution in the TAL after AKI is a protein-specific event and its increased interstitial presence negatively regulates the evolving inflammatory signaling in neighboring proximal tubules, thereby enhancing kidney recovery. The increase of serum THP may be used as a prognostic biomarker for recovery from AKI.
Figures





References
-
- Abbate M, Bonventre JV, Brown D. The microtubule network of renal epithelial cells is disrupted by ischemia and reperfusion. Am J Physiol Renal Fluid Electrolyte Physiol 267: F971–F978, 1994 - PubMed
-
- Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985 - PubMed
-
- Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65: 791–797, 2004 - PubMed
-
- Bleyer AJ, Hart TC, Shihabi Z, Robins V, Hoyer JR. Mutations in the uromodulin gene decrease urinary excretion of Tamm-Horsfall protein. Kidney Int 66: 974–977, 2004 - PubMed
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

