Central role of the exchange factor GEF-H1 in TNF-α-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells
- PMID: 23389627
- PMCID: PMC3608494
- DOI: 10.1091/mbc.E12-09-0661
Central role of the exchange factor GEF-H1 in TNF-α-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells
Abstract
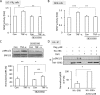
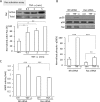
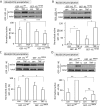
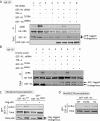
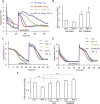
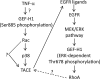
Transactivation of the epidermal growth factor receptor (EGFR) by tumor necrosis factor-α (TNF-α) is a key step in mediating RhoA activation and cytoskeleton and junction remodeling in the tubular epithelium. In this study we explore the mechanisms underlying TNF-α-induced EGFR activation. We show that TNF-α stimulates the TNF-α convertase enzyme (TACE/a disintegrin and metalloproteinase-17), leading to activation of the EGFR/ERK pathway. TACE activation requires the mitogen-activated protein kinase p38, which is activated through the small GTPase Rac. TNF-α stimulates both Rac and RhoA through the guanine nucleotide exchange factor (GEF)-H1 but by different mechanisms. EGFR- and ERK-dependent phosphorylation at the T678 site of GEF-H1 is a prerequisite for RhoA activation only, whereas both Rac and RhoA activation require GEF-H1 phosphorylation on S885. Of interest, GEF-H1-mediated Rac activation is upstream from the TACE/EGFR/ERK pathway and regulates T678 phosphorylation. We also show that TNF-α enhances epithelial wound healing through TACE, ERK, and GEF-H1. Taken together, our findings can explain the mechanisms leading to hierarchical activation of Rac and RhoA by TNF-α through a single GEF. This mechanism could coordinate GEF functions and fine-tune Rac and RhoA activation in epithelial cells, thereby promoting complex functions such as sheet migration.
Figures




Similar articles
-
The epidermal growth factor receptor mediates tumor necrosis factor-alpha-induced activation of the ERK/GEF-H1/RhoA pathway in tubular epithelium.J Biol Chem. 2011 Mar 18;286(11):9268-79. doi: 10.1074/jbc.M110.179903. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212278 Free PMC article.
-
Extracellular signal-regulated kinase and GEF-H1 mediate depolarization-induced Rho activation and paracellular permeability increase.Am J Physiol Cell Physiol. 2010 Jun;298(6):C1376-87. doi: 10.1152/ajpcell.00408.2009. Epub 2010 Mar 17. Am J Physiol Cell Physiol. 2010. PMID: 20237148 Free PMC article.
-
GEF-H1/RhoA signalling pathway mediates lipopolysaccharide-induced intercellular adhesion molecular-1 expression in endothelial cells via activation of p38 and NF-κB.Cytokine. 2012 Mar;57(3):417-28. doi: 10.1016/j.cyto.2011.12.009. Epub 2012 Jan 9. Cytokine. 2012. PMID: 22226621
-
Rac signaling in breast cancer: a tale of GEFs and GAPs.Cell Signal. 2012 Feb;24(2):353-362. doi: 10.1016/j.cellsig.2011.08.011. Epub 2011 Aug 27. Cell Signal. 2012. PMID: 21893191 Free PMC article. Review.
-
Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement.Cells. 2022 Nov 11;11(22):3565. doi: 10.3390/cells11223565. Cells. 2022. PMID: 36428994 Free PMC article. Review.
Cited by
-
Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex.FASEB J. 2014 Jul;28(7):3249-60. doi: 10.1096/fj.13-245142. Epub 2014 Apr 4. FASEB J. 2014. PMID: 24706358 Free PMC article.
-
iRhom2 promotes lupus nephritis through TNF-α and EGFR signaling.J Clin Invest. 2018 Apr 2;128(4):1397-1412. doi: 10.1172/JCI97650. Epub 2018 Mar 5. J Clin Invest. 2018. PMID: 29369823 Free PMC article.
-
S-1-propenylcysteine improves TNF-α-induced vascular endothelial barrier dysfunction by suppressing the GEF-H1/RhoA/Rac pathway.Cell Commun Signal. 2021 Feb 15;19(1):17. doi: 10.1186/s12964-020-00692-w. Cell Commun Signal. 2021. PMID: 33588881 Free PMC article.
-
Role of guanine nucleotide exchange factor-H1 in complement-mediated RhoA activation in glomerular epithelial cells.J Biol Chem. 2014 Feb 14;289(7):4206-18. doi: 10.1074/jbc.M113.506816. Epub 2013 Dec 19. J Biol Chem. 2014. PMID: 24356971 Free PMC article.
-
YAP and TAZ protect against white adipocyte cell death during obesity.Nat Commun. 2020 Oct 28;11(1):5455. doi: 10.1038/s41467-020-19229-3. Nat Commun. 2020. PMID: 33116140 Free PMC article.
References
-
- Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530–34536. - PubMed
-
- Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis. Trends Cell Biol. 2008;18:210–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

