FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants
- PMID: 23389878
- PMCID: PMC3915545
- DOI: 10.1002/pros.22649
FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants
Abstract
Background: Aberrant activation of the androgen receptor (AR) is a major factor highly relevant to castration-resistant progression of prostate cancer (PCa). FOXO1, a key downstream effector of PTEN, inhibits androgen-independent activation of the AR. However, the underlying mechanism remains elusive.
Methods: The inhibitory effect of FOXO1 on full-length and constitutively active splice variants of the AR was examined by luciferase reporter assays and real-time reverse transcription polymerase chain reaction (RT-qPCR). In vitro protein binding assays and western blot analyses were used to determine the regions in FOXO1 and AR responsible for their interaction.
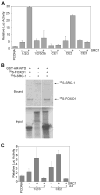
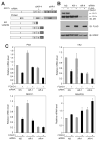
Results: We found that a putative transcription repression domain in the NH2-terminus of FOXO1 is dispensable for FOXO1 inhibition of the AR. In vitro protein binding assays showed that FOXO1 binds to the transcription activation unit 5 (TAU5) motif in the AR NH2-terminal domain (NTD), a region required for recruitment of p160 activators including SRC-1. Ectopic expression of SRC-1 augmented transcriptional activity of some, but not all AR splice variants examined. Forced expression of FOXO1 blocked the effect of SRC-1 on AR variants' transcriptional activity by decreasing the binding of SRC-1 to the AR NTD. Ectopic expression of FOXO1 inhibited expression of endogenous genes activated primarily by alternatively spliced AR variants in human castration-resistant PCa 22Rv1 cells.
Conclusions: FOXO1 binds to the TAU5 motif in the AR NTD and inhibits ligand-independent activation of AR splice variants, suggesting the PTEN/FOXO1 pathway as a potential therapeutic target for inhibition of aberrant AR activation and castration-resistant PCa growth.
Copyright © 2013 Wiley Periodicals, Inc.
Figures




References
-
- Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12:193–207. - PubMed
-
- van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–193. - PubMed
-
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. - PubMed
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. - PubMed
-
- Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

