Controlled multicenter evaluation of a bacteriophage-based method for rapid detection of Staphylococcus aureus in positive blood cultures
- PMID: 23390282
- PMCID: PMC3666813
- DOI: 10.1128/JCM.02967-12
Controlled multicenter evaluation of a bacteriophage-based method for rapid detection of Staphylococcus aureus in positive blood cultures
Abstract
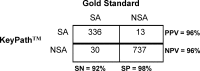
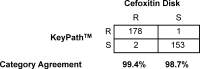
Staphylococci are a frequent cause of bloodstream infections (BSIs). Appropriate antibiotic treatment for BSIs may be delayed because conventional laboratory testing methods take 48 to 72 h to identify and characterize isolates from positive blood cultures. We evaluated a novel assay based on bacteriophage amplification that identifies Staphylococcus aureus and differentiates between methicillin-susceptible and methicillin-resistant S. aureus (MSSA and MRSA, respectively) in samples taken directly from signal-positive Bactec blood culture bottles within 24 h of positive signal, with results available within 5 h. The performance of the MicroPhage KeyPath MRSA/MSSA blood culture test was compared to conventional identification and susceptibility testing methods. At four sites, we collectively tested a total of 1,165 specimens, of which 1,116 were included in our analysis. Compared to standard methods, the KeyPath MRSA/MSSA blood culture test demonstrated a sensitivity, specificity, positive predictive value, and negative predictive value of 91.8%, 98.3%, 96.3%, and 96.1%, respectively, for correctly identifying S. aureus. Of those correctly identified as S. aureus (n = 334), 99.1% were correctly categorized as either MSSA or MRSA. Analysis of a subset of the data revealed that the KeyPath MRSA/MSSA blood culture test delivered results a median of 30 h sooner than conventional methods (a median of 46.9 h versus a median of 16.9 h). Although the sensitivity of the test in detecting S. aureus-positive samples is not high, its accuracy in determining methicillin resistance and susceptibility among positives is very high. These characteristics may enable earlier implementation of appropriate antibiotic treatment for many S. aureus BSI patients.
Figures
References
-
- Pien BC, Sundaram P, Raoof N, Costa SF, Mirrett S, Woods CW, Reller LB, Weinstein MP. 2010. The clinical and prognostic importance of positive blood cultures in adults. Am. J. Med. 123:819–828 - PubMed
-
- Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24:584–602 - PubMed
-
- Reed SD, Friedman JY, Engemann JJ, Griffiths RI, Anstrom KJ, Kaye KS, Stryjewski ME, Szczech LA, Reller LB, Corey GR, Schulman KA, Fowler VG., Jr 2005. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 26:175–183 - PubMed
-
- Gopal AK, Fowler VG, Jr, Shah M, Gesty-Palmer D, Marr KA, McClelland RS, Kong LK, Gottlieb GS, Lanclos K, Li J, Sexton DJ, Corey GR. 2000. Prospective analysis of Staphylococcus aureus bacteremia in nonneutropenic adults with malignancy. J. Clin. Oncol. 18:1110–1115 - PubMed
-
- Stryjewski ME, Szczech LA, Benjamin DK, Jr, Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR, Fowler VG., Jr 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44:190–196 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

