Chronic stress disrupts neural coherence between cortico-limbic structures
- PMID: 23390414
- PMCID: PMC3565161
- DOI: 10.3389/fncir.2013.00010
Chronic stress disrupts neural coherence between cortico-limbic structures
Abstract
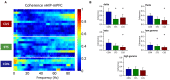
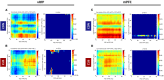
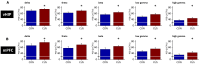
Chronic stress impairs cognitive function, namely on tasks that rely on the integrity of cortico-limbic networks. To unravel the functional impact of progressive stress in cortico-limbic networks we measured neural activity and spectral coherences between the ventral hippocampus (vHIP) and the medial prefrontal cortex (mPFC) in rats subjected to short term stress (STS) and chronic unpredictable stress (CUS). CUS exposure consistently disrupted the spectral coherence between both areas for a wide range of frequencies, whereas STS exposure failed to trigger such effect. The chronic stress-induced coherence decrease correlated inversely with the vHIP power spectrum, but not with the mPFC power spectrum, which supports the view that hippocampal dysfunction is the primary event after stress exposure. Importantly, we additionally show that the variations in vHIP-to-mPFC coherence and power spectrum in the vHIP correlated with stress-induced behavioral deficits in a spatial reference memory task. Altogether, these findings result in an innovative readout to measure, and follow, the functional events that underlie the stress-induced reference memory impairments.
Keywords: chronic stress; coherence; hippocampus; power spectrum; prefrontal cortex.
Figures




References
-
- Amat J., Paul E., Zarza C., Watkins L. R., Maier S. F. (2006). Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 26, 13264–13272 10.1523/JNEUROSCI.3630-06.2006 - DOI - PMC - PubMed
-
- Buzsáki G. (2006). Rhythms of the Brain. 1st Edn USA: Oxford University Press
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

