Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury
- PMID: 23390552
- PMCID: PMC3563583
- DOI: 10.1371/journal.pone.0055806
Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury
Abstract
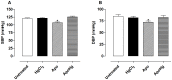
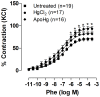
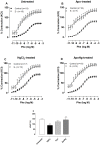
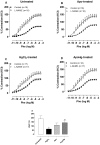
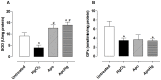
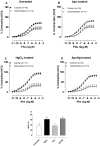
Mercury increases the risk of cardiovascular disease and oxidative stress and alters vascular reactivity. This metal elicits endothelial dysfunction causing decreased NO bioavailability via increased oxidative stress and contractile prostanoid production. NADPH oxidase is the major source of reactive oxygen species (ROS) in the vasculature. Our aim was to investigate whether treatment with apocynin, an NADPH oxidase inhibitor, prevents the vascular effects caused by chronic intoxication with low concentrations of mercury. Three-month-old male Wistar rats were treated for 30 days with a) intramuscular injections (i.m.) of saline; b) HgCl(2) (i.m. 1(st) dose: 4.6 µg/kg, subsequent doses: 0.07 µg/kg/day); c) Apocynin (1.5 mM in drinking water plus saline i.m.); and d) Apocynin plus HgCl(2). The mercury treatment resulted in 1) an increased aortic vasoconstrictor response to phenylephrine and reduced endothelium-dependent responses to acetylcholine; 2) the increased involvement of ROS and vasoconstrictor prostanoids in response to phenylephrine, whereas the endothelial NO modulation of such responses was reduced; and 3) the reduced activity of aortic superoxide dismutase (SOD) and glutathione peroxidase (GPx) and increased plasma malondialdehyde (MDA) levels. Treatment with apocynin partially prevented the increased phenylephrine responses and reduced the endothelial dysfunction elicited by mercury treatment. In addition, apocynin treatment increased the NO modulation of vasoconstrictor responses and aortic SOD activity and reduced plasma MDA levels without affecting the increased participation of vasoconstrictor prostanoids observed in aortic segments from mercury-treated rats.
Conclusions: Mercury increases the vasoconstrictor response to phenylephrine by reducing NO bioavailability and increasing the involvement of ROS and constrictor prostanoids. Apocynin protects the vessel from the deleterious effects caused by NADPH oxidase, but not from those caused by prostanoids, thus demonstrating a two-way action.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

