HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer
- PMID: 23391338
- PMCID: PMC3672694
- DOI: 10.1186/bcr3384
HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer
Abstract
Introduction: Recent data suggest that benefit from trastuzumab and chemotherapy might be related to expression of HER2 and estrogen receptor (ESR1). Therefore, we investigated HER2 and ESR1 mRNA levels in core biopsies of HER2-positive breast carcinomas from patients treated within the neoadjuvant GeparQuattro trial.
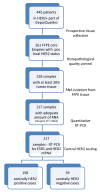
Methods: HER2 levels were centrally analyzed by immunohistochemistry (IHC), silver in situ hybridization (SISH) and qRT-PCR in 217 pretherapeutic formalin-fixed, paraffin-embedded (FFPE) core biopsies. All tumors had been HER2-positive by local pathology and had been treated with neoadjuvant trastuzumab/ chemotherapy in GeparQuattro.
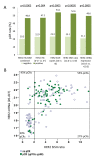
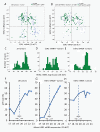
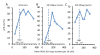
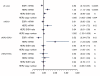
Results: Only 73% of the tumors (158 of 217) were centrally HER2-positive (cHER2-positive) by IHC/SISH, with cHER2-positive tumors showing a significantly higher pCR rate (46.8% vs. 20.3%, P <0.0005). HER2 status by qRT-PCR showed a concordance of 88.5% with the central IHC/SISH status, with a low pCR rate in those tumors that were HER2-negative by mRNA analysis (21.1% vs. 49.6%, P <0.0005). The level of HER2 mRNA expression was linked to response rate in ESR1-positive tumors, but not in ESR1-negative tumors. HER2 mRNA expression was significantly associated with pCR in the HER2-positive/ESR1-positive tumors (P = 0.004), but not in HER2-positive/ESR1-negative tumors.
Conclusions: Only patients with cHER2-positive tumors - irrespective of the method used - have an increased pCR rate with trastuzumab plus chemotherapy. In patients with cHER2-negative tumors the pCR rate is comparable to the pCR rate in the non-trastuzumab treated HER-negative population. Response to trastuzumab is correlated to HER2 mRNA levels only in ESR1-positive tumors. This study adds further evidence to the different biology of both subsets within the HER2-positive group.
Figures
References
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. - DOI - PubMed
-
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. - DOI - PMC - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. - DOI - PubMed
-
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkiö S, Möykkynen K, Helle L, Ingalsuo S, Pajunen M, Huusko M, Salminen T, Auvinen P, Leinonen H, Leinonen M, Isola J, Kellokumpu-Lehtinen PL. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel orvinorelbine with or without trastuzumab as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. - DOI - PubMed
-
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kühn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

