Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism
- PMID: 23391720
- PMCID: PMC3582134
- DOI: 10.1172/JCI65351
Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism
Abstract
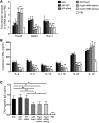
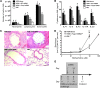
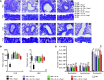
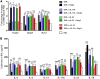
Chemically modified mRNA is capable of inducing therapeutic levels of protein expression while circumventing the threat of genomic integration often associated with viral vectors. We utilized this novel therapeutic tool to express the regulatory T cell transcription factor, FOXP3, in a time- and site-specific fashion in murine lung, in order to prevent allergic asthma in vivo. We show that modified Foxp3 mRNA rebalanced pulmonary T helper cell responses and protected from allergen-induced tissue inflammation, airway hyperresponsiveness, and goblet cell metaplasia in 2 asthma models. This protection was conferred following delivery of modified mRNA either before or after the onset of allergen challenge, demonstrating its potential as both a preventive and a therapeutic agent. Mechanistically, FOXP3 induction controlled Th2 and Th17 inflammation by regulating innate immune cell recruitment through an IL-10-dependent pathway. The protective effects of FOXP3 could be reversed by depletion of IL-10 or administration of recombinant IL-17A or IL-23. Delivery of Foxp3 mRNA to sites of inflammation may offer a novel, safe therapeutic tool for the treatment of allergic asthma and other diseases driven by an imbalance in helper T cell responses.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

