Transforming growth factor-β signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1
- PMID: 23391818
- PMCID: PMC3752402
- DOI: 10.1053/j.gastro.2013.01.056
Transforming growth factor-β signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1
Abstract
Background & aims: Transforming growth factor (TGF)-β-activated kinase 1 (TAK1) is activated in different cytokine signaling pathways. Deletion of Tak1 from hepatocytes results in spontaneous development of hepatocellular carcinoma (HCC), liver inflammation, and fibrosis. TGF-β activates TAK1 and Smad signaling, which regulate cell death, proliferation, and carcinogenesis. However, it is not clear whether TGF-β signaling in hepatocytes, via TGF-β receptor-2 (Tgfbr2), promotes HCC and liver fibrosis.
Methods: We generated mice with hepatocyte-specific deletion of Tak1 (Tak1ΔHep), as well as Tak1/Tgfbr2DHep and Tak1/Smad4ΔHep mice. Tak1flox/flox, Tgfbr2ΔHep, and Smad4ΔHep mice were used as controls, respectively. We assessed development of liver injury, inflammation, fibrosis, and HCC. Primary hepatocytes isolated from these mice were used to assess TGF-β-mediated signaling.
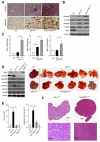
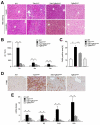
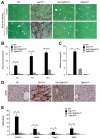
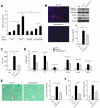
Results: Levels of TGF-β, TGF-βR2, and phospho-Smad2/3 were increased in HCCs from Tak1ΔHep mice, which developed liver fibrosis and inflammation by 1 month and HCC by 9 months. However, Tak1/Tgfbr2ΔHep mice did not have this phenotype, and their hepatocytes did not undergo spontaneous cell death or compensatory proliferation. Hepatocytes from Tak1ΔHep mice incubated with TGF-β did not activate p38, c-Jun N-terminal kinase, or nuclear factor-κB; conversely, TGF-β-mediated cell death and phosphorylation of Smad2/3 were increased, compared with control hepatocytes. Blocking the Smad pathway inhibited TGF-β-mediated death of Tak1-/- hepatocytes. Accordingly, disruption of Smad4 reduced the spontaneous liver injury, inflammation, fibrosis, and HCC that develops in Tak1ΔHep mice. Levels of the anti-apoptotic protein Bcl-xL, β-catenin, connective tissue growth factor, and vascular endothelial growth factor were increased in HCC from Tak1ΔHep mice, but not in HCCs from Tak1/Tgfbr2ΔHep mice. Injection of N-nitrosodiethylamine induced HCC formation in wild-type mice, but less in Tgfbr2ΔHep mice.
Conclusions: TGF-β promotes development of HCC in Tak1ΔHep mice by inducing hepatocyte apoptosis and compensatory proliferation during early phases of tumorigenesis, and inducing expression of anti-apoptotic, pro-oncogenic, and angiogenic factors during tumor progression.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Weber A, Boege Y, Reisinger F, et al. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly. 2011;141:w13197. - PubMed
-
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. - PubMed
-
- Bettermann K, Vucur M, Haybaeck J, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. - PubMed
-
- Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24 DK080506/DK/NIDDK NIH HHS/United States
- R01AA02172/AA/NIAAA NIH HHS/United States
- R01 AA020172/AA/NIAAA NIH HHS/United States
- P42ES010337/ES/NIEHS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 DK085252/DK/NIDDK NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- P30CA23100-27/CA/NCI NIH HHS/United States
- R01DK085252/DK/NIDDK NIH HHS/United States
- DK080506/DK/NIDDK NIH HHS/United States
- K23 DK090303/DK/NIDDK NIH HHS/United States
- K23-DK090303-2/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

