Circadian control of the immune system
- PMID: 23391992
- PMCID: PMC4090048
- DOI: 10.1038/nri3386
Circadian control of the immune system
Abstract
Circadian rhythms, which have long been known to play crucial roles in physiology, are emerging as important regulators of specific immune functions. Circadian oscillations of immune mediators coincide with the activity of the immune system, possibly allowing the host to anticipate and handle microbial threats more efficiently. These oscillations may also help to promote tissue recovery and the clearance of potentially harmful cellular elements from the circulation. This Review summarizes the current knowledge of circadian rhythms in the immune system and provides an outlook on potential future implications.
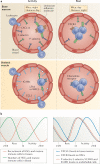
Figures


References
-
- Halberg F, Halberg E, Barnum CP, Bittner JJ. Physiological 24-hour Periodicity in Human Beings and Mice, the Lighting Regimen and Daily Routine. In: Withrow RB, editor. Photoperiodism and Related Phenomena in Plants and Animals. 1959. pp. 803–78.
-
- Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. - PubMed
-
- Storch KF, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

