ASPP1 and ASPP2 bind active RAS, potentiate RAS signalling and enhance p53 activity in cancer cells
- PMID: 23392125
- PMCID: PMC3595493
- DOI: 10.1038/cdd.2013.3
ASPP1 and ASPP2 bind active RAS, potentiate RAS signalling and enhance p53 activity in cancer cells
Abstract
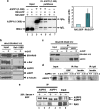
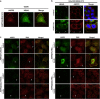
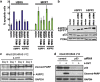
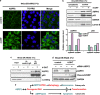
RAS mutations occur frequently in human cancer and activated RAS signalling contributes to tumour development and progression. Apart from its oncogenic effects on cell growth, active RAS has tumour-suppressive functions via its ability to induce cellular senescence and apoptosis. RAS is known to induce p53-dependent cell cycle arrest, yet its effect on p53-dependent apoptosis remains unclear. We report here that apoptosis-stimulating protein of p53 (ASPP) 1 and 2, two activators of p53, preferentially bind active RAS via their N-terminal RAS-association domains (RAD). Additionally, ASPP2 colocalises with and contributes to RAS cellular membrane localisation and potentiates RAS signalling. In cancer cells, ASPP1 and ASPP2 cooperate with oncogenic RAS to enhance the transcription and apoptotic function of p53. Thus, loss of ASPP1 and ASPP2 in human cancer cells may contribute to the full transforming property of RAS oncogene.
Figures


References
-
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev. 2003;3:11–22. - PubMed
-
- Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786:178–187. - PubMed
-
- Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol. 2011;8:492–503. - PubMed
-
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

