Lsm proteins and Hfq: Life at the 3' end
- PMID: 23392247
- PMCID: PMC3710366
- DOI: 10.4161/rna.23695
Lsm proteins and Hfq: Life at the 3' end
Abstract
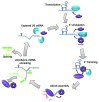
The bacterial Hfq protein is a versatile modulator of RNA function and is particularly important for regulation mediated by small non-coding RNAs. Hfq is a bacterial Sm protein but bears more similarity to the eukaryotic Sm-like (Lsm) family of proteins than the prototypical Sm proteins. Hfq and Lsm proteins share the ability to chaperone RNA-RNA and RNA/protein interactions and an interesting penchant for protecting the 3' end of a transcript from exonucleolytic decay while encouraging degradation through other pathways. Our view of Lsm function in eukaryotes has historically been informed by studies of Hfq structure and function but mutational analyses and structural studies of Lsm sub-complexes have given important insights as well. Here, we aim to compare and contrast the roles of these evolutionarily related complexes and to highlight areas for future investigation.
Keywords: RNA chaperone; Sm-like; exoribonuclease; mRNA decay; oligouridylation; polyadenylation; splicing.
Figures



References
-
- Franze de Fernandez MT, Hayward WS, August JT. Bacterial proteins required for replication of phage Q ribonucleic acid. Purification and properties of host factor I, a ribonucleic acid-binding protein. J Biol Chem. 1972;247:824–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
