Can we stop mass drug administration prior to 3 annual rounds in communities with low prevalence of trachoma?: PRET Ziada trial results
- PMID: 23392481
- PMCID: PMC3790327
- DOI: 10.1001/jamaophthalmol.2013.2356
Can we stop mass drug administration prior to 3 annual rounds in communities with low prevalence of trachoma?: PRET Ziada trial results
Abstract
Importance: The World Health Organization recommends at least 3 annual mass drug administrations (MDAs) of azithromycin in places where the prevalence of follicular trachoma (FT) is greater than 10%. However, stopping MDA prior to 3 rounds, if monitoring indicates an absence of infection with Chlamydia trachomatis even if FT persists, may be more cost-effective.
Objective: To determine the prevalence of infection in communities randomized to 3 rounds of annual MDAs with azithromycin compared with communities randomized to a stopping rule, where MDA could cease if the infection rate was low. DESIGN A 1:1 community randomized trial comparing usual care with a cessation rule. The Partnership for the Rapid Elimination of Trachoma-Ziada Trial was conducted from February 1, 2010, through September 1, 2011.
Setting: Sixteen communities in Tanzania with trachoma prevalence rates between 10% and 20%.
Participants: A total of 100 children aged 5 years or younger randomly drawn from each community. Children had to reside in an eligible community, have no ocular condition that prevented trachoma grading or ocular specimen collection, and have a guardian who could provide consent for participation.
Interventions: Cessation of MDA with azithromycin if the community had no infection in their sample at 6 months or 18 months.
Main outcome measure: The prevalence of C trachomatis at 18 months.
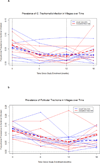
Results: None of the intervention communities met criteria to stop MDA based on the 6-month or 18-month survey; all, as well as the usual care communities, were scheduled for a third MDA round. There was no difference in infection (2.9% vs 4.7%; P = .25) between the usual care and cessation rule communities at 18 months.
Conclusions and relevance: In this setting, communities with low (10%-20%) initial prevalence of active trachoma did not have MDA stopped before 3 annual rounds on the basis of monitoring for infection. Infection with C trachomatis in communities with average trachoma rates at 12% to 13% cannot be eliminated before 3 rounds of MDA with azithromycin.
Trial registration: clinicaltrials.gov Identifier: NCT00792922.
Figures

References
-
- Cook JA. Eliminating blinding trachoma. N Engl J Med. 2008;358:1777–1779. - PubMed
-
- Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–568. - PubMed
-
- Solomon A, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. In: Trachoma control: a guide for programme managers. Organization WH, editor. Geneva, Switzerland: 2006.
-
- Stare D, Harding-Esch E, Munoz B, et al. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiol. 2011;18:20–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

