Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making
- PMID: 23392657
- PMCID: PMC3623291
- DOI: 10.1523/JNEUROSCI.2984-12.2013
Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making
Abstract
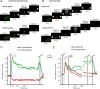
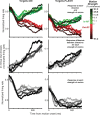
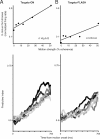
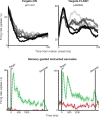
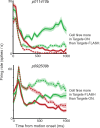
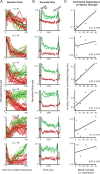
Previous work has revealed a remarkably direct neural correlate of decisions in the lateral intraparietal area (LIP). Specifically, firing rate has been observed to ramp up or down in a manner resembling the accumulation of evidence for a perceptual decision reported by making a saccade into (or away from) the neuron's response field (RF). However, this link between LIP response and decision formation emerged from studies where a saccadic target was always stimulating the RF during decisions, and where the neural correlate was the averaged activity of a restricted sample of neurons. Because LIP cells are (1) highly responsive to the presence of a visual stimulus in the RF, (2) heterogeneous, and (3) not clearly anatomically segregated from large numbers of neurons that fail selection criteria, the underlying neuronal computations are potentially obscured. To address this, we recorded single neuron spiking activity in LIP during a well-studied moving-dot direction-discrimination task and manipulated whether a saccade target was present in the RF during decision-making. We also recorded from a broad sample of LIP neurons, including ones conventionally excluded in prior studies. Our results show that cells multiplex decision signals with decision-irrelevant visual signals. We also observed disparate, repeating response "motifs" across neurons that, when averaged together, resemble traditional ramping decision signals. In sum, neural responses in LIP simultaneously carry decision signals and decision-irrelevant sensory signals while exhibiting diverse dynamics that reveal a broader range of neural computations than previously entertained.
Figures






References
-
- Ben Hamed S, Duhamel JR. Ocular fixation and visual activity in the monkey lateral intraparietal area. Exp Brain Res. 2002;142:512–528. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
