Mapping the subcellular distribution of α-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson's disease pathogenesis
- PMID: 23392688
- PMCID: PMC3711410
- DOI: 10.1523/JNEUROSCI.2898-12.2013
Mapping the subcellular distribution of α-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson's disease pathogenesis
Abstract
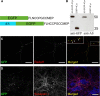
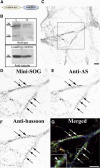
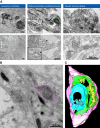
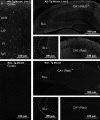
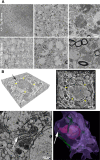
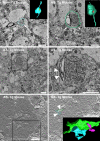
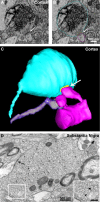
Modifications to the gene encoding human α-synuclein have been linked to the development of Parkinson's disease. The highly conserved structure of α-synuclein suggests a functional interaction with membranes, and several lines of evidence point to a role in vesicle-related processes within nerve terminals. Using recombinant fusions of human α-synuclein, including new genetic tags developed for correlated light microscopy and electron microscopy (the tetracysteine-biarsenical labeling system or the new fluorescent protein for electron microscopy, MiniSOG), we determined the distribution of α-synuclein when overexpressed in primary neurons at supramolecular and cellular scales in three dimensions (3D). We observed specific association of α-synuclein with a large and otherwise poorly characterized membranous organelle system of the presynaptic terminal, as well as with smaller vesicular structures within these boutons. Furthermore, α-synuclein was localized to multiple elements of the protein degradation pathway, including multivesicular bodies in the axons and lysosomes within neuronal cell bodies. Examination of synapses in brains of transgenic mice overexpressing human α-synuclein revealed alterations of the presynaptic endomembrane systems similar to our findings in cell culture. Three-dimensional electron tomographic analysis of enlarged presynaptic terminals in several brain areas revealed that these terminals were filled with membrane-bounded organelles, including tubulovesicular structures similar to what we observed in vitro. We propose that α-synuclein overexpression is associated with hypertrophy of membrane systems of the presynaptic terminal previously shown to have a role in vesicle recycling. Our data support the conclusion that α-synuclein is involved in processes associated with the sorting, channeling, packaging, and transport of synaptic material destined for degradation.
Figures

References
-
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
-
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. - PubMed
-
- Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103412/GM/NIGMS NIH HHS/United States
- 5P41RR004050-24/RR/NCRR NIH HHS/United States
- R25 DA026401/DA/NIDA NIH HHS/United States
- R01 GM086197-05/GM/NIGMS NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- 8 P41 GM103412-24/GM/NIGMS NIH HHS/United States
- R01 GM086197/GM/NIGMS NIH HHS/United States
- AG184440/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- R01 NS027177/NS/NINDS NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
