Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase
- PMID: 23392690
- PMCID: PMC6619186
- DOI: 10.1523/JNEUROSCI.2906-12.2013
Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase
Abstract
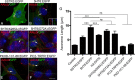
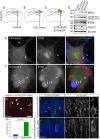
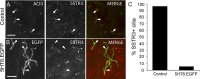
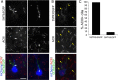
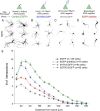
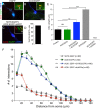
The formation of primary cilia is a highly choreographed process that can be disrupted in developing neurons by overexpressing neuromodulatory G-protein-coupled receptors GPCRs or by blocking intraflagellar transport. Here, we examined the effects of overexpressing the ciliary GPCRs, 5HT6 and SSTR3, on cilia structure and the differentiation of neocortical neurons. Neuronal overexpression of 5HT6 and SSTR3 was achieved by electroporating mouse embryo cortex in utero with vectors encoding these receptors. We found that overexpression of ciliary GPCRs in cortical neurons, especially 5HT6, induced the formation of long (>30 μm) and often forked cilia. These changes were associated with increased levels of intraflagellar transport proteins and accelerated ciliogenesis in neonatal neocortex, the induction of which required Kif3a, an anterograde motor critical for cilia protein trafficking and growth. GPCR overexpression also altered the complement of signaling molecules within the cilia. We found that SSTR3 and type III adenylyl cyclase (ACIII), proteins normally enriched in neuronal cilia, were rarely detected in 5HT6-elongated cilia. Intriguingly, the changes in cilia structure were accompanied by changes in neuronal morphology. Specifically, disruption of normal ciliogenesis in developing neocortical neurons, either by overexpressing cilia GPCRs or a dominant-negative form of Kif3a, significantly impaired dendrite outgrowth. Remarkably, coexpression of ACIII with 5HT6 restored ACIII to cilia, normalized cilia structure, and restored dendrite outgrowth, effects that were not observed in neurons coexpressing ACIII and dominant-negative form of Kif3a. Collectively, our data suggest the formation of neuronal dendrites in developing neocortex requires structurally normal cilia enriched with ACIII.
Figures

References
-
- Anastas SB, Mueller D, Semple-Rowland SL, Breunig JJ, Sarkisian MR. Failed cytokinesis of neural progenitors in citron kinase-deficient rats leads to multiciliated neurons. Cereb Cortex. 2011;21:338–344. - PubMed
-
- Bennouna-Greene V, Kremer S, Stoetzel C, Christmann D, Schuster C, Durand M, Verloes A, Sigaudy S, Holder-Espinasse M, Godet J, Brandt C, Marion V, Danion A, Dietemann JL, Dollfus H. Hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with Bardet-Biedl syndrome underline complex CNS impact of primary cilia. Clin Genet. 2011;80:523–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
