Receptive-field subfields of V2 neurons in macaque monkeys are adult-like near birth
- PMID: 23392691
- PMCID: PMC3711597
- DOI: 10.1523/JNEUROSCI.4377-12.2013
Receptive-field subfields of V2 neurons in macaque monkeys are adult-like near birth
Abstract
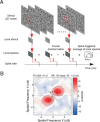
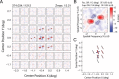
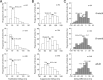
Infant primates can discriminate texture-defined form despite their relatively low visual acuity. The neuronal mechanisms underlying this remarkable visual capacity of infants have not been studied in nonhuman primates. Since many V2 neurons in adult monkeys can extract the local features in complex stimuli that are required for form vision, we used two-dimensional dynamic noise stimuli and local spectral reverse correlation to measure whether the spatial map of receptive-field subfields in individual V2 neurons is sufficiently mature near birth to capture local features. As in adults, most V2 neurons in 4-week-old monkeys showed a relatively high degree of homogeneity in the spatial matrix of facilitatory subfields. However, ∼25% of V2 neurons had the subfield map where the neighboring facilitatory subfields substantially differed in their preferred orientations and spatial frequencies. Over 80% of V2 neurons in both infants and adults had "tuned" suppressive profiles in their subfield maps that could alter the tuning properties of facilitatory profiles. The differences in the preferred orientations between facilitatory and suppressive profiles were relatively large but extended over a broad range. Response immaturities in infants were mild; the overall strength of facilitatory subfield responses was lower than that in adults, and the optimal correlation delay ("latency") was longer in 4-week-old infants. These results suggest that as early as 4 weeks of age, the spatial receptive-field structure of V2 neurons is as complex as in adults and the ability of V2 neurons to compare local features of neighboring stimulus elements is nearly adult like.
Figures







References
-
- Amir Y, Harel M, Malach R. Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual-cortex. J Comp Neurol. 1993;334:19–46. - PubMed
-
- Anzai A, Peng X, Van Essen DC. Neurons in monkey visual area V2 encode combinations of orientations. Nat Neurosci. 2007;10:1313–1321. - PubMed
-
- Atkinson J, Hood B, Wattam-Bell J, Anker S, Tricklebank J. Development of orientation discrimination in infancy. Perception. 1988;17:587–595. - PubMed
-
- Barone P, Dehay C, Berland M, Kennedy H. Role of directed growth and target selection in the formation of cortical pathways: prenatal development of the projection of area V2 to area V4 in the monkey. J Comp Neurol. 1996;374:1–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
