Mutations in the nucleolar phosphoprotein, nucleophosmin, promote the expression of the oncogenic transcription factor MEF/ELF4 in leukemia cells and potentiates transformation
- PMID: 23393136
- PMCID: PMC3611015
- DOI: 10.1074/jbc.M112.415703
Mutations in the nucleolar phosphoprotein, nucleophosmin, promote the expression of the oncogenic transcription factor MEF/ELF4 in leukemia cells and potentiates transformation
Abstract
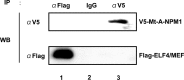
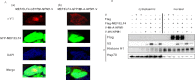
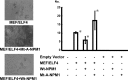
Myeloid ELF1-like factor (MEF/ELF4), a member of the ETS transcription factors, can function as an oncogene in murine cancer models and is overexpressed in various human cancers. Here, we report a mechanism by which MEF/ELF4 may be activated by a common leukemia-associated mutation in the nucleophosmin gene. By using a tandem affinity purification assay, we found that MEF/ELF4 interacts with multifactorial protein nucleophosmin (NPM1). Coimmunoprecipitation and GST pull-down experiments demonstrated that MEF/ELF4 directly forms a complex with NPM1 and also identified the region of NPM1 that is responsible for this interaction. Functional analyses showed that wild-type NPM1 inhibited the DNA binding and transcriptional activity of MEF/ELF4 on the HDM2 promoter, whereas NPM1 mutant protein (Mt-NPM1) enhanced these activities of MEF/ELF4. Induction of Mt-NPM1 into MEF/ELF4-overexpressing NIH3T3 cells facilitated malignant transformation. In addition, clinical leukemia samples with NPM1 mutations had higher human MDM2 (HDM2) mRNA expression. Our data suggest that enhanced HDM2 expression induced by mutant NPM1 may have a role in MEF/ELF4-dependent leukemogenesis.
Figures





References
-
- Miyazaki Y., Sun X., Uchida H., Zhang J., Nimer S. (1996) A novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene 13, 1721–1729 - PubMed
-
- Hedvat C. V., Yao J., Sokolic R. A., Nimer S. D. (2004) Myeloid ELF1-like factor is a potent activator of interleukin-8 expression in hematopoietic cells. J. Biol. Chem. 279, 6395–6400 - PubMed
-
- Lacorazza H. D., Miyazaki Y., Di Cristofano A., Deblasio A., Hedvat C., Zhang J., Cordon-Cardo C., Mao S., Pandolfi P. P., Nimer S. D. (2002) The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 17, 437–449 - PubMed
-
- Lu Z., Kim K. A., Suico M. A., Shuto T., Li J. D., Kai H. (2004) MEF up-regulates human β-defensin 2 expression in epithelial cells. FEBS Lett. 561, 117–121 - PubMed
-
- Seki Y., Suico M. A., Uto A., Hisatsune A., Shuto T., Isohama Y., Kai H. (2002) The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res. 62, 6579–6586 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

