Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension
- PMID: 23393185
- PMCID: PMC3612802
- DOI: 10.1210/jc.2012-3249
Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension
Abstract
Objective: This study investigated the efficacy and safety of insulin degludec (IDeg) once daily (OD), varying injection timing day to day in subjects with type 1 diabetes.
Research design and methods: This 26-week, open-label, treat-to-target, noninferiority trial compared IDeg forced flexible (Forced-Flex) OD (given in a fixed schedule with a minimum 8 and maximum 40 hours between doses) with IDeg or insulin glargine (IGlar) given at the same time daily OD. In the 26-week extension, all IDeg subjects were transferred to a free-flexible (Free-Flex) regimen, which allowed any-time-of-day dosing, and compared with subjects continued on IGlar.
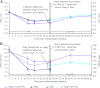
Results: After 26 treatment weeks, mean glycosylated hemoglobin was reduced with IDeg Forced- Flex (-0.40%), IDeg (-0.41%), and IGlar (-0.58%). IDeg Forced-Flex noninferiority was achieved. Fasting plasma glucose reductions were similar with IDeg Forced-Flex and IGlar but greater with IDeg (-2.54 mmol/L) than IDeg Forced-Flex (-1.28 mmol/L) (P = .021). At week 52, IDeg Free-Flex subjects had similar glycosylated hemoglobin but greater fasting plasma glucose reductions than IGlar subjects (-1.07 mmol/L) (P = .005). Confirmed hypoglycemia rates (plasma glucose <3.1 mmol/L or severe hypoglycemia) were similar at weeks 26 and 52. Nocturnal confirmed hypoglycemia was lower with IDeg Forced-Flex vs IDeg (37%; P = .003) and IGlar (40%; P = .001) at week 26 and 25% lower with IDeg Free-Flex vs IGlar (P = .026) at week 52.
Conclusions: IDeg can be administered OD at any time of day, with injection timing varied without compromising glycemic control or safety vs same-time-daily IDeg or IGlar. This may improve basal insulin adherence by allowing injection-time adjustment according to individual needs.
Trial registration: ClinicalTrials.gov NCT01079234.
Figures

References
-
- Rubin RR. 2005. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 118(suppl 5A):27S–34S - PubMed
-
- Morris AD, Boyle DIR, McMahon AD, Greene SA, MacDonald TM, Newton RW; for the DARTS/MEMO Collaboration 1997. Adherence to insulin treatment, glycemic control, and ketoacidosis in insulin-dependent diabetes mellitus. Lancet 350:1505–1510 - PubMed
-
- Anderson RT, Marrero D, Skovlund SE, Cramer J, Schwartz S. Self-reported compliance with insulin injection therapy in subjects with type 1 and type 2 diabetes. Diabetes Metab. 2003;29:A275 (Abstract)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

