Emerging mechanisms of T-tubule remodelling in heart failure
- PMID: 23393229
- PMCID: PMC3697065
- DOI: 10.1093/cvr/cvt020
Emerging mechanisms of T-tubule remodelling in heart failure
Abstract
Cardiac excitation-contraction coupling occurs primarily at the sites of transverse (T)-tubule/sarcoplasmic reticulum junctions. The orderly T-tubule network guarantees the instantaneous excitation and synchronous activation of nearly all Ca(2+) release sites throughout the large ventricular myocyte. Because of the critical roles played by T-tubules and the array of channels and transporters localized to the T-tubule membrane network, T-tubule architecture has recently become an area of considerable research interest in the cardiovascular field. This review will focus on the current knowledge regarding normal T-tubule structure and function in the heart, T-tubule remodelling in the transition from compensated hypertrophy to heart failure, and the impact of T-tubule remodelling on myocyte Ca(2+) handling function. In the last section, we discuss the molecular mechanisms underlying T-tubule remodelling in heart disease.
Figures
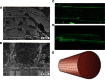
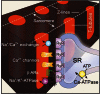
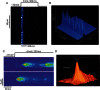
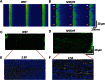


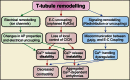
Comment in
-
Imaging T-tubules: dynamic membrane structures for deep functions.Cardiovasc Res. 2013 May 1;98(2):162-4. doi: 10.1093/cvr/cvt058. Epub 2013 Mar 14. Cardiovasc Res. 2013. PMID: 23504549 Free PMC article. No abstract available.
References
-
- Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. Boston: Kluwer Academic Publishers; 2001.
-
- Stern MD. Theory of excitation–contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi:10.1016/S0006-3495(92)81615-6. - DOI - PMC - PubMed
-
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi:10.1038/35069083. - DOI - PubMed
-
- Sun XH, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–671. doi:10.1083/jcb.129.3.659. - DOI - PMC - PubMed
-
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi:10.1152/physrev.00030.2007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

