SPAK differentially mediates vasopressin effects on sodium cotransporters
- PMID: 23393317
- PMCID: PMC3582200
- DOI: 10.1681/ASN.2012040404
SPAK differentially mediates vasopressin effects on sodium cotransporters
Abstract
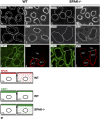
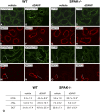
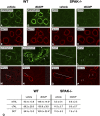
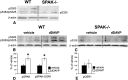
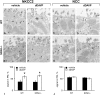
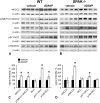
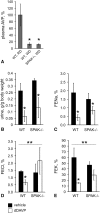
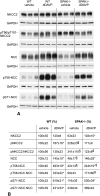
Activation of the Na(+)-K(+)-2Cl(-)-cotransporter (NKCC2) and the Na(+)-Cl(-)-cotransporter (NCC) by vasopressin includes their phosphorylation at defined, conserved N-terminal threonine and serine residues, but the kinase pathways that mediate this action of vasopressin are not well understood. Two homologous Ste20-like kinases, SPS-related proline/alanine-rich kinase (SPAK) and oxidative stress responsive kinase (OSR1), can phosphorylate the cotransporters directly. In this process, a full-length SPAK variant and OSR1 interact with a truncated SPAK variant, which has inhibitory effects. Here, we tested whether SPAK is an essential component of the vasopressin stimulatory pathway. We administered desmopressin, a V2 receptor-specific agonist, to wild-type mice, SPAK-deficient mice, and vasopressin-deficient rats. Desmopressin induced regulatory changes in SPAK variants, but not in OSR1 to the same degree, and activated NKCC2 and NCC. Furthermore, desmopressin modulated both the full-length and truncated SPAK variants to interact with and phosphorylate NKCC2, whereas only full-length SPAK promoted the activation of NCC. In summary, these results suggest that SPAK mediates the effect of vasopressin on sodium reabsorption along the distal nephron.
Figures


References
-
- Gamba G: Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 - PubMed
-
- Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP: Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 - PubMed
-
- Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 - PubMed
-
- Uchida S: Pathophysiological roles of WNK kinases in the kidney. Pflugers Arch 460: 695–702, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

