Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease
- PMID: 23393413
- PMCID: PMC3553572
- DOI: 10.31887/DCNS.2012.14.4/gbuzsaki
Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease
Abstract
The perpetual activity of the cerebral cortex is largely supported by the variety of oscillations the brain generates, spanning a number of frequencies and anatomical locations, as well as behavioral correlates. First, we review findings from animal studies showing that most forms of brain rhythms are inhibition-based, producing rhythmic volleys of inhibitory inputs to principal cell populations, thereby providing alternating temporal windows of relatively reduced and enhanced excitability in neuronal networks. These inhibition-based mechanisms offer natural temporal frames to group or "chunk" neuronal activity into cell assemblies and sequences of assemblies, with more complex multi-oscillation interactions creating syntactical rules for the effective exchange of information among cortical networks. We then review recent studies in human psychiatric patients demonstrating a variety alterations in neural oscillations across all major psychiatric diseases, and suggest possible future research directions and treatment approaches based on the fundamental properties of brain rhythms.
La perpetua actividad de la corteza cerebral está sustentada en gran medida por la variedad de oscilaciones que genera el cerebro, las que abarcan un número de frecuencias y sitios anatómicos, así como correlatos conductuales. En primer lugar se revisan los hallazgos de estudios animales que muestran que la mayoría de las formas de los ritmos cerebrales se basan en la inhibición, produciendo descargas rítmicas de estímulos inhibitorios a las principales poblaciones celulares, proporcionando por lo tanto ventanas temporales que alternan una excitabilidad relativamente reducida o aumentada en los circuitos neuronales. Estos mecanismos basados en la inhibición ofrecen marcos temporales naturales para agrupar o “fragmentar” la actividad neuronal en conjuntos celulares y secuencias de conjuntos, con interacciones más complejas de multi-oscilación creando reglas sintácticas para el cambio efectivo de información entre los circuitos corticales. Luego se revisan los estudios en pacientes psiquiátricos que demuestran una variedad de alteraciones en las oscilaciones neurales en las principales enfermedades psiquiátricas, y sugieren posibles orientaciones en las investigaciones a futuro y aproximaciones terapéuticas basadas en las propiedades fundamentals de los ritmos cerebrales.
L'activité permanente du cortex cérébral est largement basée sur la grande variété d'oscillations que le cerveau génère incluant un grand nombre de fréquences et de localisations anatomiques, ainsi que leurs corrélats comportementaux. Nous présentons tout d'abord les recherches sur les études animales montrant que la plupart des formes des rythmes cérébraux sont basées sur l'inhibition, produisant des volées rythmiques de signaux inhibiteurs vers les populations cellulaires principales, fournissant alors des fenêtres temporales alternatives d'excitabilité relativement réduites et plus importantes dans les réseaux neuronaux. Ces mécanismes inhibiteurs offrent des cadres temporaux naturels à une grosse activité neuronale ou activité groupée dans des ensembles de cellules et des séquences d'ensembles de cellules, avec des interactions multi-oscillatoires plus complexes créant des règles syntaxiques pour l'échange efficace d'information parmi les réseaux corticaux. Nous analysons ensuite des études récentes de patients psychiatriques qui montrent des altérations variées des oscillations neurales dans toutes les principales maladies psychiatriques. De possibles directions de recherche future ainsi que des approches de traitement fondées sur les propriétés fondamentales des rythmes cérébraux sont proposées.
Keywords: action potential; assembly; brain; coding; neuron; oscillation; psychiatry.
Figures
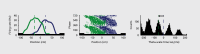
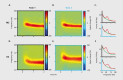
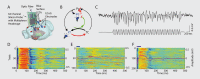
References
-
- Dehaene S., Changeux J-P. Experimental and theoretical approaches to conscious processing.. Neuron.. 2011;70:200–227. - PubMed
-
- Tononi G., Edelman GM. Consciousness and complexity.. Science. 1998;282:1846–1851. - PubMed
-
- Sherrington CS. Man On His Nature.New York, NY; Cambridge, UK: The Macmillan Company; The University Press. 1941
-
- Varela F., Lachaux J-P., Rodriguez E., Martinerie J. The brainweb phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. - PubMed
-
- Engel AK., Fries P., Singer W. Dynamic predictions oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
