Lipid-like Nanoparticles for Small Interfering RNA Delivery to Endothelial Cells
- PMID: 23393492
- PMCID: PMC3564673
- DOI: 10.1002/adfm.200900519
Lipid-like Nanoparticles for Small Interfering RNA Delivery to Endothelial Cells
Abstract
Here we develop nanoparticles composed of lipid-like materials (lipidoids) to facilitate non-viral delivery of small interfering RNA (siRNA) to endothelial cells (ECs). Nanoparticles composed of siRNA and lipidoids with small size (~200 nm) and positive charge (~34 mV) were formed by self assembly of lipidoids and siRNA. Ten lipidoids were synthesized and screened for their ability to facilitate the delivery of siRNA into ECs. Particles composed of leading lipidoids show significantly better delivery to ECs than a leading commercially-available transfection reagent, Lipofectamine 2000. As a model of potential therapeutic application, nanoparticles composed of the top performing lipidoid, NA114, were studied for their ability to deliver siRNA targeting anti-angiogenic factor (SHP-1) to human ECs. Silencing of SHP-1 expression significantly enhanced EC proliferation and decreased EC apoptosis under a simulated ischemic condition.
Figures



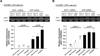


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
