Clinical symptoms and risk factors in cerebral microangiopathy patients
- PMID: 23393549
- PMCID: PMC3564848
- DOI: 10.1371/journal.pone.0053455
Clinical symptoms and risk factors in cerebral microangiopathy patients
Abstract
Objective: Although the clinical manifestation and risk factors of cerebral microangiopathy (CM) remain unclear, the number of diagnoses is increasing. Hence, patterns of association among lesion topography and severity, clinical symptoms and demographic and disease risk factors were investigated retrospectively in a cohort of CM patients.
Methods: Patients treated at the Department of Neurology, University of Bonn for CM (n = 223; 98m, 125f; aged 77.32±9.09) from 2005 to 2010 were retrospectively enrolled. Clinical symptoms, blood chemistry, potential risk factors, demographic data and ratings of vascular pathology in the brain based on the Wahlund scale were analyzed using Pearson's chi square test and one-way ANOVA.
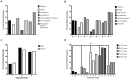
Results: Progressive cognitive decline (38.1%), gait apraxia (27.8%), stroke-related symptoms and seizures (24.2%), TIA-symptoms (22%) and vertigo (17%) were frequent symptoms within the study population. Frontal lobe WMLs/lacunar infarcts led to more frequent presentation of progressive cognitive decline, seizures, gait apraxia, stroke-related symptoms, TIA, vertigo and incontinence. Parietooccipital WMLs/lacunar infarcts were related to higher frequencies of TIA, seizures and incontinence. Basal ganglia WMLs/lacunar infarcts were seen in patients with more complaints of gait apraxia, vertigo and incontinence. Age (p = .012), arterial hypertension (p<.000), obesity (p<.000) and cerebral macroangiopathy (p = .018) were positively related to cerebral lesion load. For increased glucose level, homocysteine, CRP and D-Dimers there was no association.
Conclusion: This underlines the association of CM with neurological symptoms upon admission in a topographical manner. Seizures and vertigo are symptoms of CM which may have been missed in previous studies. In addition to confirming known risk factors such as aging and arterial hypertension, obesity appears to increase the risk as well. Since the incidence of CM is increasing, future studies should focus on the importance of prevention of vascular risk factors on its pathogenesis.
Conflict of interest statement
Figures


References
-
- Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9: 689–701 doi:10.1016/S1474-4422(10)70104-6. - DOI - PubMed
-
- Pantoni L, Garcia JH (1997) Cognitive impairment and cellular/vascular changes in the cerebral white matter. Ann N Y Acad Sci 826: 92–102. - PubMed
-
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, et al. (2011) Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatr 82: 126–135 doi:10.1136/jnnp.2009.204685. - DOI - PubMed
-
- Patel B, Markus HS (2011) Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke 6: 47–59 doi:10.1111/j.1747-4949.2010.00552.x. - DOI - PubMed
-
- Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, et al. (2008) Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 70: 935–942 doi:10.1212/01.wnl.0000305959.46197.e6. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

