Pathophysiology of NASH: perspectives for a targeted treatment
- PMID: 23394092
- PMCID: PMC3984586
- DOI: 10.2174/13816128113199990344
Pathophysiology of NASH: perspectives for a targeted treatment
Abstract
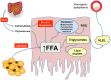
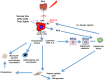
Non alcoholic steatohepatitis (NASH) is the more severe form of nonalcoholic fatty liver disease. In NASH, fatty liver, hepatic inflammation, hepatocyte injury and fibrogenesis are associated, and this condition may eventually lead to cirrhosis. Current treatment of NASH relies on the reduction of body weight and increase in physical activity, but there is no pharmacologic treatment approved as yet. Emerging data indicate that NASH progression results from parallel events originating from the liver as well as from the adipose tissue, the gut and the gastrointestinal tract. Thus, dysfunction of the adipose tissue through enhanced flow of free fatty acids and release of adipocytokines, and alterations in the gut microbiome generate proinflammatory signals that underlie NASH progression. Additional 'extrahepatic hits' include dietary factors and gastrointestinal hormones. Within the liver, hepatocyte apoptosis, ER stress and oxidative stress are key contributors to hepatocellular injury. In addition, lipotoxic mediators and danger signals activate Kupffer cells which initiate and perpetuate the inflammatory response by releasing inflammatory mediators that contribute to inflammatory cell recruitment and development of fibrosis. Inflammatory and fibrogenic mediators include chemokines, the cannabinoid system, the inflammasome and activation of pattern-recognition receptors. Here we review the major mechanisms leading to appearance and progression of NASH, focusing on both extrahepatic signals and local inflammatory mechanisms, in an effort to identify the most promising molecular targets for the treatment of this condition.
Conflict of interest statement
None
Figures



References
-
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. - PubMed
-
- Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–9. - PubMed
-
- Roulot D, Costes J-L, Buyck J-Fo, Warzocha U, Gambier N, Czernichow Sb, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011 in press. - PubMed
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. - PubMed
-
- Ong J, Younossi ZM, Reddy V, Price LL, Gramlich T, Mayes J, et al. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797–801. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

