Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment
- PMID: 23394097
- PMCID: PMC3850262
- DOI: 10.2174/13816128113199990381
Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment
Abstract
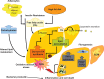
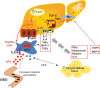
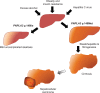
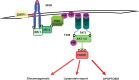
Liver fat deposition related to systemic insulin resistance defines non-alcoholic fatty liver disease (NAFLD) which, when associated with oxidative hepatocellular damage, inflammation, and activation of fibrogenesis, i.e. non-alcoholic steatohepatitis (NASH), can progress towards cirrhosis and hepatocellular carcinoma. Due to the epidemic of obesity, NAFLD is now the most frequent liver disease and the leading cause of altered liver enzymes in Western countries. Epidemiological, familial, and twin studies provide evidence for an element of heritability of NAFLD. Genetic modifiers of disease severity and progression have been identified through genome-wide association studies. These include the Patatin-like phosholipase domain-containing 3 (PNPLA3) gene variant I148M as a major determinant of inter-individual and ethnicity-related differences in hepatic fat content independent of insulin resistance and serum lipid concentration. Association studies confirm that the I148M polymorphism is also a strong modifier of NASH and progressive hepatic injury. Furthermore, a few large multicentre case-control studies have demonstrated a role for genetic variants implicated in insulin signalling, oxidative stress, and fibrogenesis in the progression of NAFLD towards fibrosing NASH, and confirm that hepatocellular fat accumulation and insulin resistance are key operative mechanisms closely involved in the progression of liver damage. It is now important to explore the molecular mechanisms underlying these associations between gene variants and progressive liver disease, and to evaluate their impact on the response to available therapies. It is hoped that this knowledge will offer further insights into pathogenesis, suggest novel therapeutic targets, and could help guide physicians towards individualised therapy that improves clinical outcome.
Figures

References
-
- Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. - PubMed
-
- Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–10. - PubMed
-
- Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. - PubMed
-
- Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. - PubMed
-
- Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

